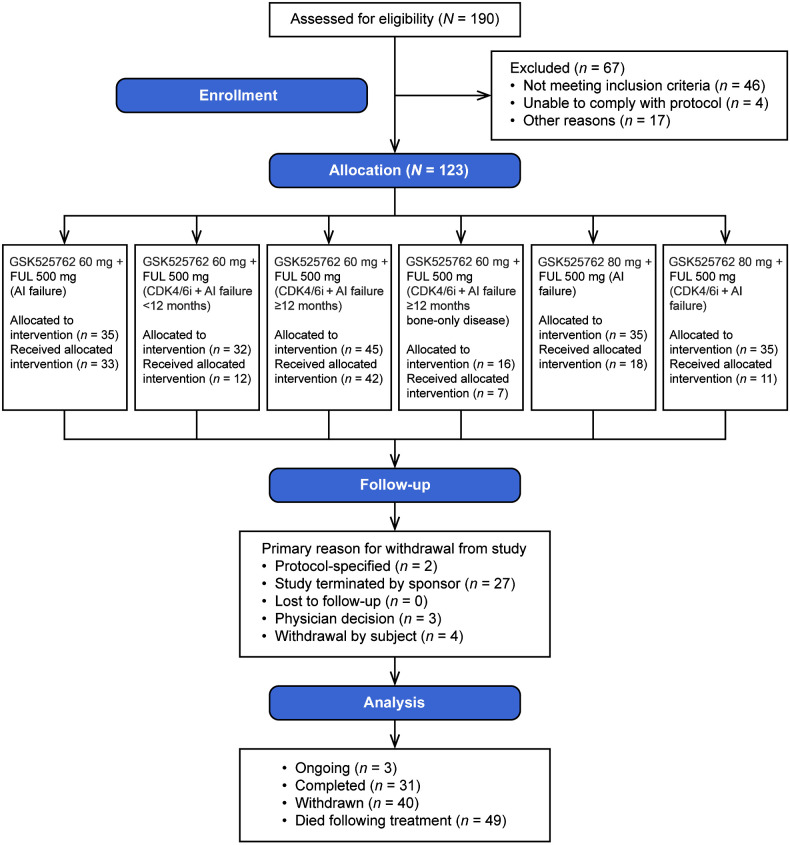
Figure 1.
CONSORT diagram for study 201973. Allocation to the GSK525762 60 mg + FUL 500 mg (CDK4/6i + AI failure ≥12 months bone-only disease), GSK525762 80 mg + FUL 500 mg (AI failure), and GSK525762 80 mg + FUL 500 mg (CDK4/6i + AI failure) groups was low because enrollment time was not adequate before the termination of the study. This is because the bone-only disease group was added later in the study, and the 80-mg cohort was stopped early; high rates of treatment discontinuation due to AE resulted in the 80-mg dose being judged to be nontolerable. AI, aromatase inhibitor; CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; FUL, fulvestrant.