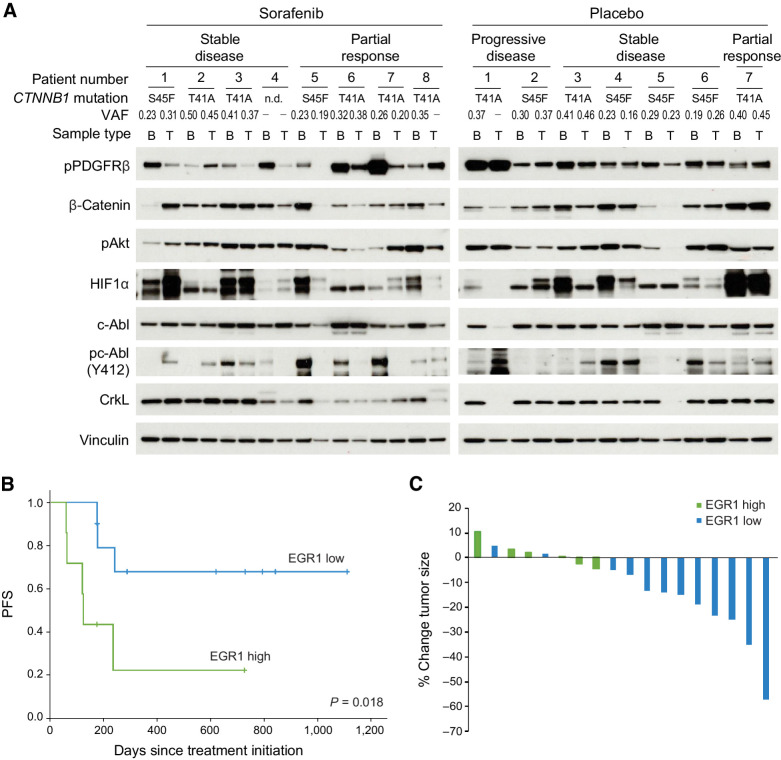
Figure 5.
Inhibition of PDGFRβ/c-Abl signaling may correlate with response to sorafenib in desmoid patients. A, Activation of PDGFRβ, c-Abl, and Akt and expression of HIF1α and β-catenin as detected by immunoblot in baseline (B) and post-treatment (T) biopsies in individual patients treated on the placebo and sorafenib arms of Alliance A091105. Best response (partial response, stable or progressive disease) as assayed by RECIST criteria or clinical progression, CTNNB1 mutations and their variant allele frequencies (VAF; identified by RNA-seq) are annotated. Samples with no mutations detected are annotated (-). B, PFS defined by clinical progression or radiographic increase in size (>15% above baseline) in patients and stratified according to high (≥50% of desmoid cells) or low (<50% of cells positive) levels of nuclear EGR1 staining on IHC of baseline samples obtained from patients in the placebo arm of the trial. C, Percent change in tumor size after four to five cycles of sorafenib in patients on Alliance A091105. Tumors with ≥90% cells staining for nuclear EGR1 are designated as EGR1 high as opposed to low.