Abstract
BACKGROUND
Fibromyalgia is a syndrome characterized by generalized chronic pain and tenderness in specific areas. Photobiomodulation therapy (PBMT) using low-level laser therapy and/or light emitting diode therapy is an electrophysical agent that can be used alone or together with a static magnetic field (PBMT-sMF) to promote analgesia in several health conditions. Little evidence exists regarding the effects of using PBMT and PBMT-sMF in patients with fibromyalgia; this evidence is conflicting.
AIM
We aimed to investigate the effects of using PBMT-sMF versus a placebo on reduction of the degree-of-pain rating, impact of fibromyalgia, pain intensity, and satisfaction with treatment in patients with fibromyalgia.
DESIGN
A prospectively registered, monocentric, randomized placebo-controlled trial, with blinding of patients, therapists, and assessors, was performed.
SETTING
The study was conducted at the Laboratory of Phototherapy and Innovative Technologies in Health (LaPIT) in Brazil, between March and October 2020.
POPULATION
Ninety female patients with fibromyalgia were randomized to undergo either PBMT-sMF (N.=45) or placebo (N.=45) treatment.
METHODS
Patients from both groups received nine treatment sessions, three times a week, for 3 weeks. Clinical outcomes were collected at baseline, the end of treatment, and at the follow-up appointment 4 weeks post-treatment. The primary outcome was the degree-of-pain rating, measured by the reduction of the tender point count.
RESULTS
A decrease in the degree-of-pain rating was observed in patients allocated to the PBMT-sMF group, decreasing the number of tender points when compared to placebo group at the end of treatment (P<0.0001) and at the follow-up assessment (P<0.0001). Patients did not report any adverse events.
CONCLUSIONS
PBMT-sMF is superior to placebo, supporting its use in patients with fibromyalgia.
CLINICAL REHABILITATION IMPACT
PBMT-sMF might be considered an important adjuvant to the treatment regimens of patients with fibromyalgia.
Keywords: Key words: Fibromyalgia; Low-level light therapy; Rheumatic diseases, Rehabilitation; Physical therapy modalities
Fibromyalgia is one of the most common rheumatic diseases and affects from 1% to 9% of the general population.1-3 Fibromyalgia is a complex syndrome characterized by generalized chronic pain and tenderness in specific areas, called tender points.4 In addition, neurological, muscular, gastrointestinal, and genitourinary symptoms5-11 are often associated with fibromyalgia, leading to a decrease in quality of life. Usually, diagnosis of fibromyalgia is complex and time consuming, and it is based on patient history, clinical examination, and exclusion of other diseases.12 Treatment is challenging, since no consistently effective treatment is available for this condition. However, several interventions are prescribed to relieve symptoms and improve physical capacity and quality of life.13 Among the non-pharmacological treatments available, photobiomodulation therapy (PBMT)14 has been shown to promote pain reduction in patients with fibromyalgia.
PBMT is an electrophysical agent that uses light amplification by stimulated emission of radiation (LASER) and light-emitting diodes (LEDs) to interact with the endogenous chromophores and promote stimulatory or inhibitory biological responses.15 In some devices, PBMT can be combined with a static magnetic field (PBMT-sMF) to improve the positive effects.16, 17 PBMT and PBMT-sMF trigger biological responses in different tissues, promoting modulation of inflammation, wound healing, bone healing, tissue regeneration, and analgesia.15 This variety of responses and effects enables PBMT and PBMT-sMF to be applied for treating different health conditions.14, 18-23
Recently, the effects of PBMT and PBMT-sMF on fibromyalgia have been investigated. Although a small clinical trial that combined PBMT and an exercise program reported evidence of negative effects,24 a systematic review demonstrated the advantages of using PBMT to treat patients with fibromyalgia.14 However, the authors pointed out the low-to-middle methodological quality of the selected studies and the large degree of heterogeneity among the trials, which demonstrated the need for further studies of high methodological quality in the field. This study showed that PBMT-sMF combined with an exercise program also showed effectiveness in reducing the pain intensity in patients with fibromyalgia.25 However, this was the first and only clinical trial to do so, and it used a small sample size to investigate the effects of the concurrent use of PBMT and a static magnetic field in patients with fibromyalgia.
Treatment with PBMT and PBMT-sMF show promise for treating pain in patients with fibromyalgia, despite the conflicting evidence; therefore, continual advance in the field, through high quality and adequately powered, randomized, controlled trials, is important. We aimed to investigate the effects of PBMT-sMF versus placebo treatment on reduction in the degree-of-pain rating, impact of fibromyalgia, pain intensity, and satisfaction with treatment in patients with fibromyalgia.
Materials and methods
Design
This was a monocentric, superiority, parallel randomized, placebo-controlled, triple-blinded (patients, therapists, and assessors) trial. This trial was prospectively registered with ClinicalTrials.gov (registration number: NCT04322812) and approved by the Research Ethics Committee of Nove de Julho University (approval number: 2.732.062). This trial was conducted in accordance with the principles set forth in the Declaration of Helsinki and written informed consent was obtained from all participants (Supplementary Digital Material 1: Supplementary Table I). No changes were made to the original protocol during the trial.
Setting and participants
This trial was carried out at the Laboratory of Phototherapy and Innovative Technologies in Health (LaPIT), São Paulo, Brazil. The inclusion criteria were as follows: female, aged between 30 and 55 years, a diagnosis of fibromyalgia that met the current criteria of the American College of Rheumatology,12 symptoms of fibromyalgia present for at least 3 months, a Widespread Pain Index (WPI) score of ≥9, a Visual Analogue Scale (VAS) score of ≥50, a Fibromyalgia Impact Questionnaire (FIQ) score of ≥50, height between 150 and 180 cm, body mass between 50 and 80 kg, a body mass index of ≥18.5 kg/m2, having an ovulatory cycle, having a sufficient level of cognition to understand procedures and to follow the guidelines.
The exclusion criteria were having arthritis, chronic fatigue syndrome, lupus, any autoimmune diseases, cognitive changes, or a pacemaker; being a sport and physical activity practitioner, injured in the last 6 months, or pregnant; having diabetes mellitus, uncontrolled blood pressure, psychiatric illness, malignant tumors, or hypersensitivity to light; or presenting with dengue, Zika virus disease, or Chikungunya in the last year.
Randomization
A researcher who was not involved in participant recruitment, assessment, or treatment conducted simple randomization with an allocation ratio of 1:1 using the website random.org. The same researcher coded the treatment according to the randomization schedule and programmed the PBMT-sMF device into active or placebo mode without disclosing the treatment allocation to anyone involved in the study. Allocation concealment was achieved using sequentially numbered, sealed, opaque envelopes. After completing their baseline assessments, eligible participants were referred to the therapist, who conducted the randomization allocation to assign them to one of two groups: the PBMT-sMF or placebo groups.
Blinding
The assessors, therapists, and patients were blinded. The assessors of the study were unaware of a patient’s allocation and the PBMT-sMF device was preprogrammed to active or placebo mode. The sounds emitted from, and information displayed on, the device’s screen were identical, regardless of the programmed mode. In addition, the device used had no thermal effects,26 enabling the blinding of the therapists and patients to be maintained throughout the treatment. The efficacy of the blinding was evaluated at the end of the study by asking assessors, therapists, and patients to guess the patient’s group allocation.
Interventions
Treatment was performed three times a week – with an interval of approximately 48 hours between sessions – for 3 consecutive weeks, yielding nine treatment sessions. The FibroLux™ Therapy System (Multi-Radiance Medical, Solon, OH, USA) was used in the treatment of both groups. The specifications of the interventions were as follows.
Active PBMT-sMF
A multiwavelength FibroLux™ (Multi-Radiance Medical) comprising four super-pulsed infrared lasers (905 nm), eight infrared LEDs (850 nm), and eight red LEDs (630 nm) was used. PBMT-sMF was applied only to the regions of the body with pain on the day of treatment, as evaluated using the Widespread Pain Index (WPI). The number of regions comprised a minimum of three and a maximum of 18 regions. The application time was 120 seconds per region, that is, the total time varied according to the number of regions treated that day. A dose of around 60 J per region was applied, that is, the total dose could vary between patients and in each session. PBMT-sMF was applied using the direct contact method, with a physical therapist applying light pressure to the skin. Table I details the parameters used.
Table I. — PBMT-sMF parameters.
| Parameter (unit) | Value or method |
|---|---|
| Number of lasers | 4 |
| Wavelength (nm) | 905 |
| Frequency (Hz) | 1000 |
| Peak power (W) – each | 50 |
| Average mean optical output (mW) – each | 5 |
| Power density (mW/cm2) – each | 15.62 |
| Energy density (J/cm2) – each | 1.87 |
| Dose (J) – each | 0.60 |
| Spot size of laser (cm2) – each | 0.32 |
| Number of red LEDs | 8 |
| Wavelength (nm) | 630 |
| Frequency (Hz) | 2 |
| Average optical output (mW) – each | 25 |
| Power density (mW/cm2) – each | 29.41 |
| Energy density (J/cm2) -each | 3.53 |
| Dose (J) – each | 3.00 |
| Spot size of red LED (cm2) – each | 0.85 |
| Number of infrared LEDs | 8 |
| Wavelength (nm) | 850 |
| Frequency (Hz) | 1000 |
| Average optical output (mW) – each | 37.50 |
| Power density (mW/cm2) – each | 66.96 |
| Energy density (J/cm2) – each | 8.04 |
| Dose (J) – each | 4.50 |
| Spot size of red LED (cm2) – each | 0.56 |
| Magnetic field (mT) | 110 |
| Irradiation time per site (s) | 120 |
| Total dose per site (J) | 62.40 |
| Aperture of device (cm2) | 30 |
| Application mode | Direct skin contact and slight pressure |
PBMT-sMF: photobiomodulation therapy combined with static magnetic field.
Placebo PBMT-sMF
The procedure for placebo PBMT-sMF treatment was similar to that of the active PBMT-sMF treatment. The same device was used; however, the 905 nm laser diodes, 850 nm LED diodes, and static magnetic field were deactivated, and the power of the 630 nm LED diodes was turned down to 1 mW (mean power for each diode) so as to keep the visual aspect of red light without delivering an effective therapeutic dose. The dose applied was lower than 1 J per region. Placebo PBMT-sMF was also applied only to the regions with pain on the day of treatment (evaluated according to the Widespread Pain Index), which could comprise a minimum of three and a maximum of 18 regions. The placebo treatment was also applied using a direct contact method, with a physical therapist applying light pressure to the skin.
Outcomes
Clinical outcomes were collected at baseline, the end of treatment (3 weeks after randomization), and the follow-up appointment 4 weeks posttreatment (7 weeks after randomization). The primary outcome was the degree-of-pain rating. The secondary outcomes included the impact of fibromyalgia, pain intensity, patient satisfaction, and adverse events. The degree-of-pain rating was indicated by the measured reduction in the tender point count, that is, the regions where the patients experienced pain, as reported using the FIQ.27 The FIQ assesses disease severity and functional ability in patients with fibromyalgia through 19 questions in ten items. The impact of fibromyalgia on each patient was measured by the FIQ score, which could range from 0 (no impact) to 100 (great impact). Pain intensity was measured using a VAS, which assessed the patient’s perceived pain levels. The VAS ranged from 0 mm (no pain) to 100 mm (worst pain imaginable). Patients’ satisfaction with treatment was measured using a Likert Scale ranging from 1 (not at all satisfied) to 5 (very satisfied). The cointerventions across the study were reported in the participant daily diary, filled by the patients. Finally, the adverse effects were recorded through reports.
Procedures
This study was divided into four separate phases: 1) phase 1 – stabilization phase; 2) phase 2 – assessment phase; 3) phase 3 – procedure phase; and 4) phase 4 – post-procedure phase. In phase 1, patients were screened to confirm their eligibility. After confirming their eligibility, the participants were invited to participate and signed a consent form. Subsequently, data regarding demographic and clinical characteristics were collected. Thereafter, pain management stabilization was performed for 1 week. At the beginning of this period, an individualized pain management regimen was determined for each participant comprising the details of medications and therapies currently used to manage fibromyalgia. Patients were allowed to maintain their pain management regimen throughout the study. However, they were asked to record it in a participant daily diary. The participants were instructed to record the intensity of pain using the VAS daily during this phase.
In phase 2, patients were screened for the last of the inclusion criteria, namely intensity of pain in the last 3 days of phase 1 (≥50), Widespread Pain Index (≥9), and FIQ Score (≥50). The baseline assessment was performed, and phase 3 began.
Phase 3 took place over 3 consecutive weeks, during which patients underwent PBMT-sMF or placebo treatment. Data regarding the primary and secondary outcomes were collected 15 minutes after the final treatment session.
In phase 4, patients returned to the Laboratory of Phototherapy and Innovative Technologies in Health (São Paulo, Brazil) for a final follow-up assessment 4 weeks after completing Phase 3. Primary and secondary outcomes data were collected, and the participant daily diary was collected.
Sample size
An individual patient success criterion is defined as a 20% or more reduction in the tender point count of where pain is experienced, as reported on the FIQ. A clinically relevant change is considered to be a 15% to 20% (approximately 10 to 15 points) reduction in the total FIQ Score.8 Assuming a unilateral alternative (that is, the intervention reduces the impact of fibromyalgia), we can detect differences of at least 15% with a power of 95% and a P value of 0.05 with two groups (intervention and usual placebo-control group) of 45 participants each, with a mean in the FIQ of approximately 70 points, and a standard deviation of approximately 20 points. Therefore, we recruited a total of 90 women with fibromyalgia (45 patients per group).
Statistical analysis
Statistical analysis was conducted following intention-to-treat principles.28 The primary statistical method to analyze the primary endpoint was Fischer’s Exact Test for two independent groups to compare the proportion of successes between groups. An unpaired t-test was applied to analyze pain intensity through the VAS. For patient satisfaction, measured through a Likert Scale, the data was reduced to the nominal level by combining all agree and disagree responses into two categories of “accept” and “reject.” The χ2 test was used after this transformation. Regarding the cointerventions, Fisher’s Exact Test was used to compare the proportions between groups. Efficacy of blinding was analyzed using Fischer’s exact categorical analysis technique to compare the proportion of successes and failures. The level of statistical significance was set at P<0.05. Data are presented in absolute values and percentage of change.
Results
The study included 90 participants with fibromyalgia divided into the placebo (45 women; mean age, 46.96 years, SD=8.11) and PBMT-sMF (45 women; mean age, 45.53 years, SD=7.95) groups between March and October 2020. The baseline characteristics of both groups were similar (age, P=0.403; weight, P=0.992; height, P=0.071) (Table II). All patients were recruited and completed all procedures between March and October 2020 (Figure 1). All patients received treatment according to the treatment allocation.
Table II. — Demographic and clinical characteristics of participants at baseline (N.=90).
| Variables | PBMT-sMF (N.=45) | Placebo (N.=45) |
|---|---|---|
| Sex | ||
| Female | 45 (100) | 45 (100) |
| Age (y) | 45.53 (7.95) | 46.96 (8.11) |
| Weight (kg) | 70.11 (9.62) | 70.13 (11.52) |
| Height (cm) | 160.40 (6.60) | 162.80 (5.83) |
| Degree of pain rating – TPC | 15.29 (3.08) | 15.20 (2.69) |
| Impact of fibromyalgia (0-100) | 79.68 (11.05) | 77.54 (11.57) |
| Pain intensity (0-100) | 80.64 (13.99) | 74.89 (13.54) |
Categorical variables are expressed as number (%). Continuous variables are expressed as mean (SD). PBMT-sMF: photobiomodulation therapy combined with static magnetic field.
Figure 1.
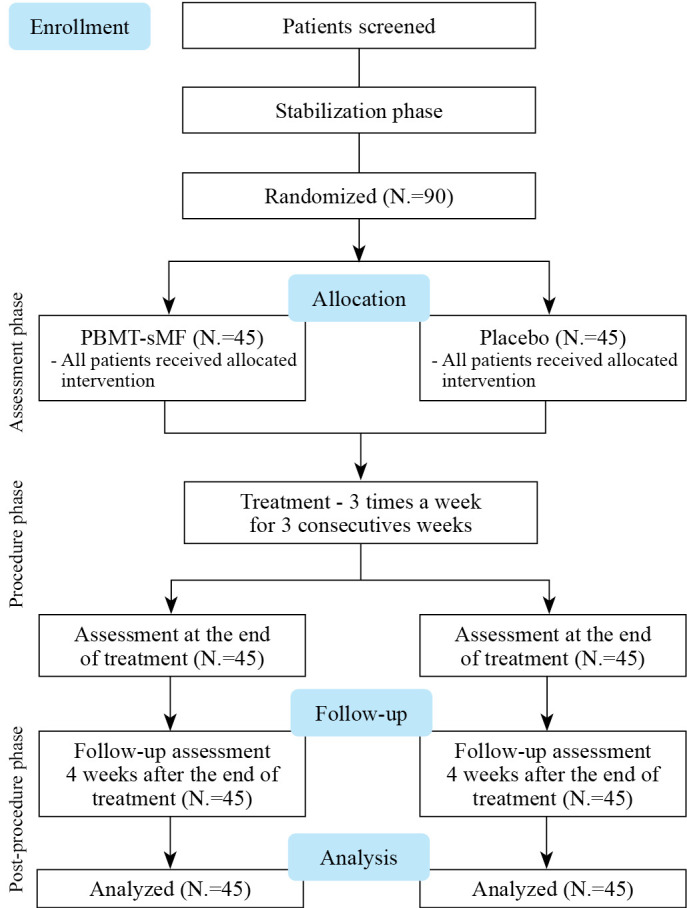
—Flow diagram of the study. PBMT-sMF: photobiomodulation therapy combined with static magnetic field.
We observed that, compared to the placebo group, patients in the PBMT-sMF group had a greater change in the degree-of-pain rating and a decreased number of tender points at the end of treatment (P<0.0001) and at the follow-up assessment (P<0.0001) (Figure 2). A between-group difference was observed in the change in impact of fibromyalgia, as measured by the total FIQ scores, in favor of the PBMT-sMF group at the end of treatment (P<0.001) and at the follow-up assessment (P=0.001) (Figure 3). In addition, the groups showed differences in terms of change in pain intensity, in favor of the PBMT-sMF group at the end of treatment (P<0.0001) and at the follow-up assessment (P<0.0001) (Figure 4). Table III shows the mean and standard deviation (absolute values) for these outcomes.
Figure 2.
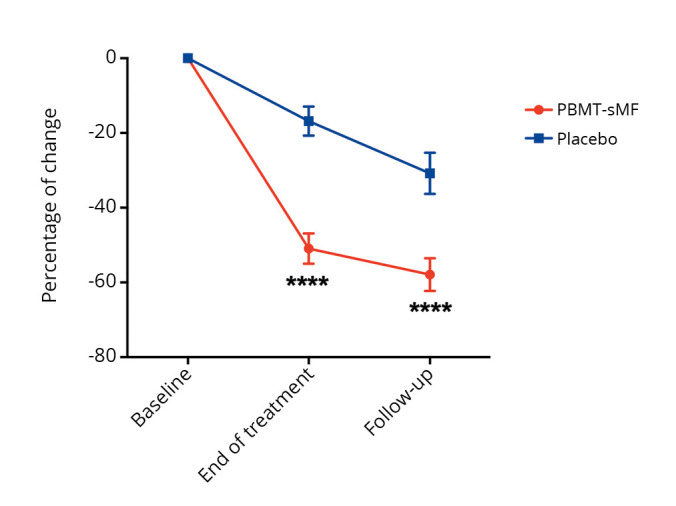
—Change in degree of pain rating, measured by the reduction of the TPC. PBMT-sMF: photobiomodulation therapy combined with static magnetic field. ***PBMT-sMF compared to placebo (P<0.0001).
Figure 3.
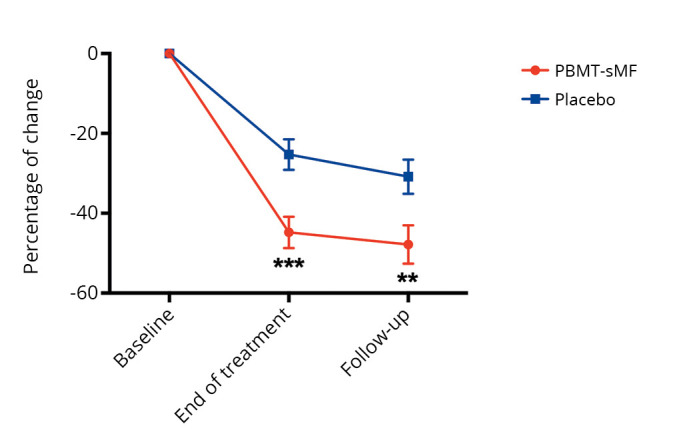
—Change in impact of fibromyalgia, measured by total FIQ scores. FIQ: Fibromyalgia Impact Questionnaire; PBMT-sMF: photobiomodulation therapy combined with static magnetic field. ***PBMT-sMF compared to placebo (P<0.001) and **PBMT-sMF compared to placebo (P<0.01).
Figure 4.
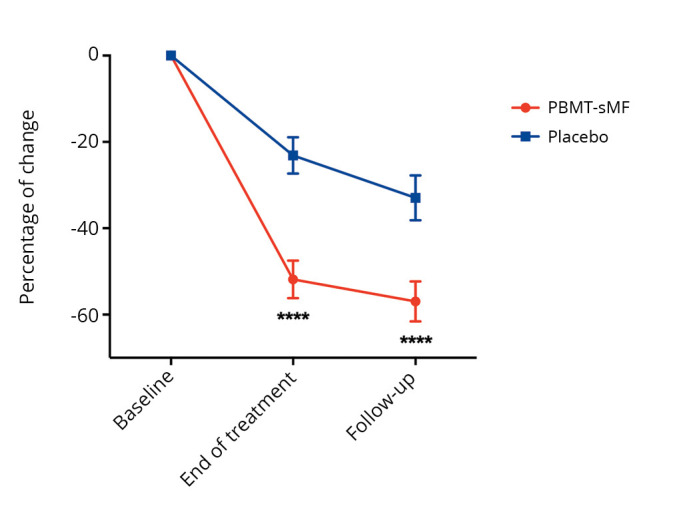
—Change in pain intensity, measured by VAS. VAS: Visual Analogue Scale; PBMT-sMF: photobiomodulation therapy combined with static magnetic field. ***PBMT-sMF compared to placebo (P<0.0001).
Table III. — Mean and standard deviation (absolute values) for the outcomes of the study (N.=90).
| Outcomes | PBMT-sMF (N.=45) | Placebo (N.=45) |
|---|---|---|
| Degree of pain rating (TPC) | ||
| Stabilization | 13.77 (3.31) | 12.07 (3.63) |
| Baseline | 15.29 (3.08) | 15.20 (2.69) |
| End of treatment | 7.29 (3.99)**** | 12.49 (3.92) |
| Follow-up | 6.22 (4.25)**** | 10.13 (4.66) |
| Impact of fibromyalgia (FIQ 0-100) | ||
| Baseline | 79.68 (11.05) | 77.54 (11.57) |
| End of treatment | 43.89 (22.34)** | 56.71 (18.63) |
| Follow-up | 41.64 (25.86)* | 52.61 (21.57) |
| Pain intensity (VAS 0-100) | ||
| Stabilization | 76.56 (17.51) | 77.37 (11.86) |
| Baseline | 80.64 (13.99) | 74.89 (13.54) |
| End of treatment | 37.80 (23.31)**** | 56.91 (20.31) |
| Follow-up | 34.47 (26.34)** | 49.58 (26.21) |
PBMT-sMF: photobiomodulation therapy combined with static magnetic field; VAS: Visual Analogue Scale; FIQ: Fibromyalgia Impact Questionnaire. Difference of placebo: ****P<0.0001; **P<0.01; *P<0.05.
Patient satisfaction at the end of treatment (P=0.00076) showed a difference between the groups: 44 (97.8%) patients in the PBMT-sMF group and 32 (71.1%) patients in the placebo group reported being “satisfied.” In contrast, no difference in patient satisfaction was found between the groups at the follow-up assessment (P=0.0513): 41 (91.1%) patients in the PBMT-sMF group and 33 (73.3%) patients in the placebo group reported being “satisfied” (Table IV).
Table IV. — Patient satisfaction with treatment in both treatment groups (N.=90).
| Parameters | End of treatment | Follow-up | ||
|---|---|---|---|---|
| PBMT-sMF (N.=45) |
Placebo (N.= 45) |
PBMT-sMF (N.=45) |
Placebo (N.=45) |
|
| Very satisfied | 36 (80) | 23 (51) | 34 (76) | 26 (58) |
| Somewhat satisfied | 8 (18) | 9 (20) | 7 (15) | 7 (15) |
| Neither satisfied nor dissatisfied | 0 (0) | 7 (15) | 4 (9) | 6 (13) |
| Not very satisfied | 1 (2) | 3 (7) | 0 (0) | 3 (7) |
| Not at all satisfied | 0 (0) | 3 (7) | 0 (0) | 3 (7) |
Categorical variables are expressed as number (%). Continuous variables are expressed as mean (SD). PBMT-sMF: photobiomodulation therapy combined with static magnetic field.
Patients reported the cointerventions during the stabilization, procedure, and post-procedure phases (Table V). The most frequent cointervention observed was the use of medication in the stabilization (80.00%), procedure (82.22%), and post-procedure (78.89%) phases. Non-opioid analgesics were the most used medications in the stabilization (45.56%), procedure (48.89%), and post-procedure (47.78%) phases. Finally, massage was the most frequently used of the other cointerventions in the stabilization (17.78%), procedure (18.89%), and post-procedure (13.33%) phases. A difference between groups in favor of the PBMT-sMF group at the procedure and post-procedure phases (P=0.0027 and P=0.0032, respectively) was found among the other cointerventions.
Table V. — Proportion of cointerventions in both treatment groups (N.=90).
| Parameters | Medication | Other cointerventions | ||||
|---|---|---|---|---|---|---|
| Time points | PBMT-sMF (N.=45) |
Placebo (N.=45) |
P | PBMT-sMF (N.=45) |
Placebo (N.=45) |
P |
| Stabilization | 34 (75.56) | 38 (84.44) | 0.4299 | 19 (42.22) | 23 (51.11) | 0.5264 |
| Procedure | 35 (77.78) | 39 (86.67) | 0.4089 | 12 (26.67)** | 27 (60.00) | 0.0027 |
| Post-procedure | 33 (73.33) | 38 (84.44) | 0.3016 | 5 (11.11)** | 18 (40.00) | 0.0032 |
Categorical variables are expressed as number (%). PBMT-sMF: photobiomodulation therapy combined with static magnetic field. Difference of placebo: **P<0.01.
To measure the efficacy of blinding, assessors, therapists, and patients were asked about the allocation of patients to treatment groups at the follow-up assessment. The assessors correctly guessed the allocation of 32 (71.1%) patients in the PBMT-sMF group and 21 (46.7%) patients in the placebo group. The therapists correctly guessed the allocation of 32 (71.1%) patients in the PBMT-sMF group and 19 (42.22%) patients in the placebo group. Thirty-eight (84.44%) and 31 (31.1%) patients guessed their allocation in the PBMT-sMF and placebo groups, respectively. No difference (P=0.134 for the assessors and P=0.05 for the patients) was found in the proportion of correct guesses in the determination of patients’ group allocation, evidencing that assessors, therapists, and patients were blinded during the study.
Patients reported no major adverse effects. However, seven (7.8%) patients reported increased pain or tension. No adverse event required any intervention or resulted in a participant withdrawing or being withdrawn from the study; each adverse effect was fully and satisfactorily resolved by study completion.
Discussion
This triple-blinded randomized controlled trial investigated the effects of the PBMT-sMF on patients with fibromyalgia. At the end of treatment and at the follow-up assessment, we observed a reduction in the degree-of-pain rating, with a reduction in the number of tender points, in patients allocated to the PBMT-sMF group. Moreover, we observed that patients allocated to the PBMT-sMF group presented with a lower impact of fibromyalgia and a greater reduction in pain intensity at the end of treatment and at the follow-up assessment than patients in the placebo group did. Finally, we observed a difference in patient satisfaction between the groups, in favor of the PBMT-sMF group, at the end of treatment; however, this difference was not sustained 1 month later.
A previous study investigated the effects of PBMT on alleviating pain and reducing the number of tender points, as well as other outcomes, in patients with fibromyalgia.29 Similar to our trial, the results of that study showed that applying PBMT for 3 minutes to each tender point (2 J per point) daily for 2 weeks enabled a decrease in pain intensity and the total number of tender points. Another controlled trial investigated the effects of using a whole-body PBMT bed to treat patients with fibromyalgia.30 As shown by the results of our trial, this study observed a reduction in pain with the use of PBMT three times weekly; the total dose administered was 1,160,400 J. Despite differences in PBMT parameters, sites of application, and frequency of treatment among the studies, their results corroborate ours, suggesting that applying PBMT may provide effective treatment for patients with fibromyalgia.
Another trial investigated the effects of administering a combination treatment regimen of PBMT alone with an exercise program that included warm-up, neuromuscular, and neuromotor exercises, and stretching in patients with fibromyalgia.24 Outcomes such as pain, functional performance, quality of life, and depression were measured. In contrast to the results of our study, this trial demonstrated that when PBMT was applied after exercise, the treatment outcomes were not better than placebo. Despite regions of pain being experienced elsewhere in the body, in this trial PBMT was only applied to the lower limbs. A total of 64 J per limb was irradiated and the treatment was performed three times per week for 8 weeks. In contrast, in our trial, PBMT was applied to those regions that were painful on the day of treatment. A total of 60 J per region was irradiated and the treatment was also performed three times a week; however, treatment only took place over 3 weeks. Additionally, in our trial we investigated the effects of PBMT-sMF, not PBMT alone in combination with an exercise program. Finally, we must highlight that, in contrast to ours, this trial had a small sample size (22 patients divided into two groups), an important 28% loss to follow-up, randomized more patients than proposed in the sample size calculation, and did not perform intention-to-treat analysis. These differences between the two trials may explain the differences found in their results. Moreover, similar to the results observed in our trial, a systematic review and meta-analysis14 demonstrated that with the use of PBMT, FIQ scores improved, severity of pain decreased, and the number of tender points decreased in patients with fibromyalgia.
Only one previous trial of the use of PBMT-sMF in patients with fibromyalgia has been performed.25 The trial investigated the effects of a single session of PBMT-sMF and those of 10 weeks of treatment. The results demonstrated that a single session of PBMT-sMF improved the pain threshold in patients with fibromyalgia. Additionally, PBMT-sMF and PBMT-sMF applied before an exercise program (PBMT-sMF plus exercise) reduced pain intensity and the number of tender points after 10 weeks of treatment. Improved FIQ scores were also observed in the PBMT-sMF and PBMT-sMF plus exercise groups after 10 weeks of treatment. These results were similar to ours; however, the protocols used for PBMT-sMF were different. In the abovementioned trial, PBMT-sMF was applied to 10 tender points, reported for pain in all patients, with a dose of 39.3 J per site. The treatment was performed two times per week over 10 weeks. In addition, PBMT-sMF was applied before an exercise protocol consisting of stretching and aerobics. In contrast to our trial, the sample size was smaller (80 patients divided into four groups), no placebo group was included, and outcomes such as pain threshold and quality of life were measured. The similarity in the results of both trials suggests and reinforces that PBMT-sMF can be effective in treating patients with fibromyalgia.
The results of our trial demonstrate that PBMT-sMF is superior to placebo, supporting its use in patients with fibromyalgia. These results can be explained by the analgesic effect of PBMT and PBMT-sMF.31 Evidence shows that PBMT causes changes in neuronal physiology and morphology, such as nerve function inhibition, nociceptor-specific inhibition, and local conduction block.32 Therefore, inhibition of neural function could be one of the mechanisms through which pain relief is promoted in patients with fibromyalgia. Regarding the dose of PBMT-sMF, taking into account the results of our and the previously25 reported trial, it may be possible to begin stablishing a possible therapeutic window (ranging from 39.3 J to 62.4 J per site irradiated) in which patients with fibromyalgia can be treated. The abovementioned trial also reported positive results using lower doses of irradiation and treating patients for longer periods than our trial did. These aspects may suggest that applying higher doses inside the above proposed therapeutic window might result in shortened durations of treatment.
Limitations of the study
Limitations of our study included measuring the effects of PBMT-sMF only in the short-term and using only females as an inclusion criterion. In addition, this was a monocentric study. The main strength of our study is its high methodological quality, showing characteristics of prospective registration, and use of true randomization, allocation concealment, and intention-to-treat analysis. The blinding efficacy was measured for assessors, therapists, and patients to ensure that the study was triple-blinded. We used a placebo group to control for confounding factors. Moreover, we controlled for cointerventions through a participant daily diary, which was maintained through the trial to avoid memory bias. Finally, no loss to follow-up occurred in this trial. Although we observed a short-term advantage of PBMT-sMF in the treatment of patients with fibromyalgia, further studies investigating its effects in the medium- and long-term are needed. Large trials that combine PBMT-sMF with an exercise program are important to ensure advancements in the field. Finally, further studies are needed to establish the PBMT-sMF dosages within the therapeutic window for fibromyalgia.
Conclusions
The findings from this study indicate that PBMT-sMF, using 62.4 J per irradiated site, three times a week, for 3 consecutive weeks, has advantage in the treatment of patients with fibromyalgia. In the short term, the use of such therapy reduces the degree-of-pain rating, impact of fibromyalgia, and pain intensity.
Supplementary Digital Material 1
Supplementary Table I
CONSORT 2010 checklist of information to include when reporting a randomized trial.*
Footnotes
Conflicts of interest :Ernesto Cesar Pinto Leal-Junior received research support from Multi-Radiance Medical (Solon, OH, USA), a laser device manufacturer. He also received a grant (grant number: 310281/2017–2) from the Brazilian Council of Science and Technology Development (the Brazilian Council of Science and Technology Development had no role in the planning, conducting, and analysis of the data of this randomized controlled trial). Douglas Scott Johnson is an employee and shareholder of Multi-Radiance Medical (Solon, OH, USA). Shaiane Silva Tomazoni has a personal relationship with Ernesto Cesar Pinto Leal-Junior.
References
- 1.Jones GT, Atzeni F, Beasley M, Flüß E, Sarzi-Puttini P, Macfarlane GJ. The prevalence of fibromyalgia in the general population: a comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis Rheumatol 2015;67:568–75. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25323744&dopt=Abstract 10.1002/art.38905 [DOI] [PubMed] [Google Scholar]
- 2.Prescott E, Kjøller M, Jacobsen S, Bülow PM, Danneskiold-Samsøe B, Kamper-Jørgensen F. Fibromyalgia in the adult Danish population: I. A prevalence study. Scand J Rheumatol 1993;22:233–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8235493&dopt=Abstract 10.3109/03009749309095129 [DOI] [PubMed] [Google Scholar]
- 3.Heidari F, Afshari M, Moosazadeh M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol Int 2017;37:1527–39. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28447207&dopt=Abstract 10.1007/s00296-017-3725-2 [DOI] [PubMed] [Google Scholar]
- 4.Gracely RH, Grant MA, Giesecke T. Evoked pain measures in fibromyalgia. Best Pract Res Clin Rheumatol 2003;17:593–609. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12849714&dopt=Abstract 10.1016/S1521-6942(03)00036-6 [DOI] [PubMed] [Google Scholar]
- 5.Rehm SE, Koroschetz J, Gockel U, Brosz M, Freynhagen R, Tölle TR, et al. A cross-sectional survey of 3035 patients with fibromyalgia: subgroups of patients with typical comorbidities and sensory symptom profiles. Rheumatology (Oxford) 2010;49:1146–52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20236955&dopt=Abstract 10.1093/rheumatology/keq066 [DOI] [PubMed] [Google Scholar]
- 6.Sandıkçı SC, Özbalkan Z. Fatigue in rheumatic diseases. Eur J Rheumatol 2015;2:109–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27708942&dopt=Abstract 10.5152/eurjrheum.2015.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kleinman L, Mannix S, Arnold LM, Burbridge C, Howard K, McQuarrie K, et al. Assessment of sleep in patients with fibromyalgia: qualitative development of the fibromyalgia sleep diary. Health Qual Life Outcomes 2014;12:111. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25017455&dopt=Abstract 10.1186/s12955-014-0111-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskelet Disord 2007;8:27. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17349056&dopt=Abstract 10.1186/1471-2474-8-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ifergane G, Buskila D, Simiseshvely N, Zeev K, Cohen H. Prevalence of fibromyalgia syndrome in migraine patients. Cephalalgia 2006;26:451–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16556247&dopt=Abstract 10.1111/j.1468-2982.2005.01060.x [DOI] [PubMed] [Google Scholar]
- 10.Mathieu N. [Somatic comorbidities in irritable bowel syndrome: fibromyalgia, chronic fatigue syndrome, and interstitial cystitis]. Gastroenterol Clin Biol 2009;33(Suppl 1):S17–25. [French] https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19303534&dopt=Abstract 10.1016/S0399-8320(09)71521-0 [DOI] [PubMed] [Google Scholar]
- 11.Nickel JC, Tripp DA, Pontari M, Moldwin R, Mayer R, Carr LK, et al. Interstitial cystitis/painful bladder syndrome and associated medical conditions with an emphasis on irritable bowel syndrome, fibromyalgia and chronic fatigue syndrome. J Urol 2010;184:1358–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20719340&dopt=Abstract 10.1016/j.juro.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62:600–10. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20461783&dopt=Abstract 10.1002/acr.20140 [DOI] [PubMed] [Google Scholar]
- 13.Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Häuser W, Fluß E, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis 2017;76:318–28. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27377815&dopt=Abstract 10.1136/annrheumdis-2016-209724 [DOI] [PubMed] [Google Scholar]
- 14.Yeh SW, Hong CH, Shih MC, Tam KW, Huang YH, Kuan YC. Low-Level Laser Therapy for Fibromyalgia: A Systematic Review and Meta-Analysis. Pain Physician 2019;22:241–54. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31151332&dopt=Abstract [PubMed] [Google Scholar]
- 15.Anders JJ, Lanzafame RJ, Arany PR. Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg 2015;33:183–4. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25844681&dopt=Abstract 10.1089/pho.2015.9848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedmann H, Lipovsky A, Nitzan Y, Lubart R. Combined magnetic and pulsed laser fields produce synergistic acceleration of cellular eléctron transfer. Laser Ther 2009;18:137–41. 10.5978/islsm.18.137 [DOI] [Google Scholar]
- 17.Eccles NK. A critical review of randomized controlled trials of static magnets for pain relief. J Altern Complement Med 2005;11:495–509. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15992236&dopt=Abstract 10.1089/acm.2005.11.495 [DOI] [PubMed] [Google Scholar]
- 18.Casalechi HL, Dumont AJ, Ferreira LA, de Paiva PR, Machado CD, de Carvalho PT, et al. Acute effects of photobiomodulation therapy and magnetic field on functional mobility in stroke survivors: a randomized, sham-controlled, triple-blind, crossover, clinical trial. Lasers Med Sci 2020;35:1253–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31782023&dopt=Abstract 10.1007/s10103-019-02898-y [DOI] [PubMed] [Google Scholar]
- 19.Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 2009;374:1897–908. [Erratum in: Lancet. 2010;375:894] https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19913903&dopt=Abstract 10.1016/S0140-6736(09)61522-1 [DOI] [PubMed] [Google Scholar]
- 20.Sobral AP, Sobral SS, Campos TM, Horliana AC, Fernandes KP, Bussadori SK, et al. Photobiomodulation and myofascial temporomandibular disorder: systematic review and meta-analysis followed by cost-effectiveness analysis. J Clin Exp Dent 2021;13:e724–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34306537&dopt=Abstract 10.4317/jced.58084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langella LG, Casalechi HL, Tomazoni SS, Johnson DS, Albertini R, Pallotta RC, et al. Photobiomodulation therapy (PBMT) on acute pain and inflammation in patients who underwent total hip arthroplasty-a randomized, triple-blind, placebo-controlled clinical trial. Lasers Med Sci 2018;33:1933–40. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29909435&dopt=Abstract 10.1007/s10103-018-2558-x [DOI] [PubMed] [Google Scholar]
- 22.Sæbø H, Naterstad IF, Joensen J, Stausholm MB, Bjordal JM. Pain and Disability of Conservatively Treated Distal Radius Fracture: A Triple-Blinded Randomized Placebo-Controlled Trial of Photobiomodulation Therapy. Photobiomodul Photomed Laser Surg 2022;40:33–41. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35030040&dopt=Abstract 10.1089/photob.2021.0125 [DOI] [PubMed] [Google Scholar]
- 23.M A . Ummer V S, Maiya AG, Hande M. Low level laser therapy for the patients with painful diabetic peripheral neuropathy - A systematic review. Diabetes Metab Syndr 2019;13:2667–70. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31405692&dopt=Abstract 10.1016/j.dsx.2019.07.035 [DOI] [PubMed] [Google Scholar]
- 24.Germano Maciel D, Trajano da Silva M, Rodrigues JA, Viana Neto JB, de França IM, Melo AB, et al. Low-level laser therapy combined to functional exercise on treatment of fibromyalgia: a double-blind randomized clinical trial. Lasers Med Sci 2018;33:1949–59. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29931588&dopt=Abstract 10.1007/s10103-018-2561-2 [DOI] [PubMed] [Google Scholar]
- 25.da Silva MM, Albertini R, de Tarso Camillo de Carvalho P, Leal-Junior EC, Bussadori SK, Vieira SS, et al. Randomized, blinded, controlled trial on effectiveness of photobiomodulation therapy and exercise training in the fibromyalgia treatment. Lasers Med Sci 2018;33:343–51. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29170901&dopt=Abstract 10.1007/s10103-017-2388-2 [DOI] [PubMed] [Google Scholar]
- 26.Grandinétti VS, Miranda EF, Johnson DS, de Paiva PR, Tomazoni SS, Vanin AA, et al. The thermal impact of phototherapy with concurrent super-pulsed lasers and red and infrared LEDs on human skin. Lasers Med Sci 2015;30:1575–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25987340&dopt=Abstract 10.1007/s10103-015-1755-0 [DOI] [PubMed] [Google Scholar]
- 27.Rosado ML, Pereira JP, da Fonseca JP, Branco JC. [Cultural adaptation and validation of the “Fibromyalgia Impact Questionnaire”—portuguese version]. Acta Reumatol Port 2006;31:157–65. [Portuguese] https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17058362&dopt=Abstract [PubMed] [Google Scholar]
- 28.Elkins MR, Moseley AM. Intention-to-treat analysis. J Physiother 2015;61:165–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26096012&dopt=Abstract 10.1016/j.jphys.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 29.Gür A, Karakoç M, Nas K, Cevik R, Saraç J, Demir E. Efficacy of low power laser therapy in fibromyalgia: a single-blind, placebo-controlled trial. Lasers Med Sci 2002;17:57–61. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11845369&dopt=Abstract 10.1007/s10103-002-8267-4 [DOI] [PubMed] [Google Scholar]
- 30.Navarro-Ledesma S, Carroll J, Burton P, Ana GM. Short-Term Effects of Whole-Body Photobiomodulation on Pain, Quality of Life and Psychological Factors in a Population Suffering from Fibromyalgia: A Triple-Blinded Randomised Clinical Trial. Pain Ther 2023;12:225–39. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36369323&dopt=Abstract 10.1007/s40122-022-00450-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen RJ. Physical agents used in the management of chronic pain by physical therapists. Phys Med Rehabil Clin N Am 2006;17:315–45. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16616270&dopt=Abstract 10.1016/j.pmr.2005.12.007 [DOI] [PubMed] [Google Scholar]
- 32.Chow RT, Armati PJ. Photobiomodulation: Implications for Anesthesia and Pain Relief. Photomed Laser Surg 2016;34:599–609. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27419354&dopt=Abstract 10.1089/pho.2015.4048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
CONSORT 2010 checklist of information to include when reporting a randomized trial.*


