Fig 2.
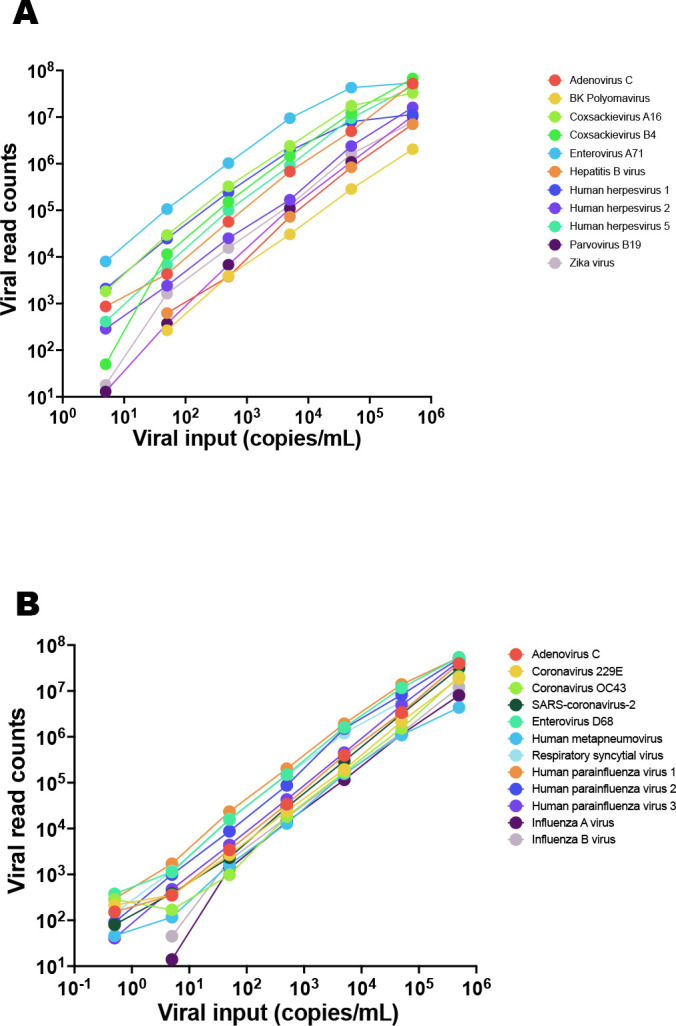
Linearity data for (A) systemic and (B) respiratory infection test validation. Virus stock was serially diluted in sample matrix (plasma or nasal swabs in VTM, respectively) that had been tested negative for the viruses used for validation, prior to processing for VirCapSeq-VERT.
