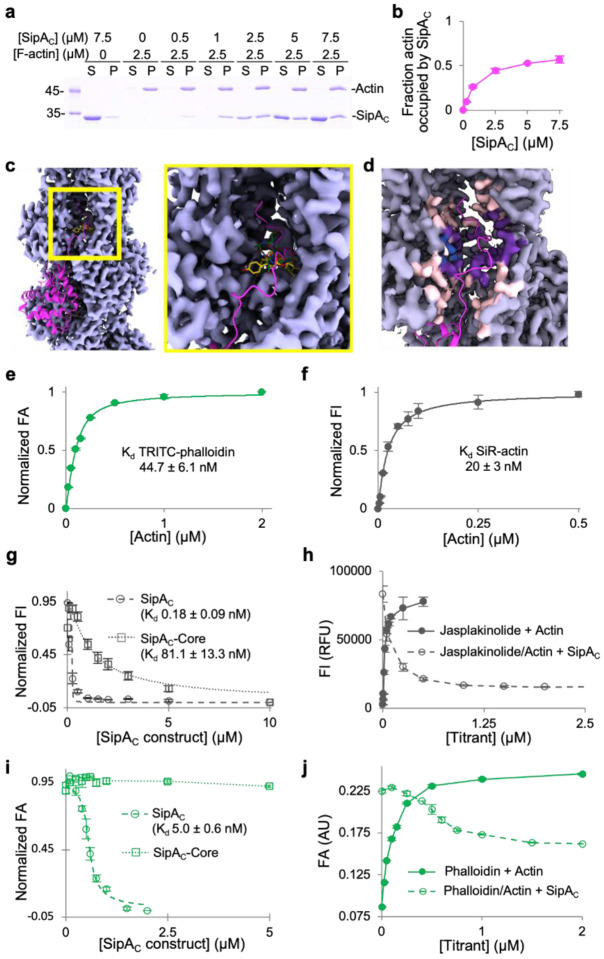
Fig. 3. Arm2’s binding site on F-actin overlaps with those of phalloidin and jasplakinolide.
a,b, Binding stoichiometry of SipAC to F-actin was determined in co-sedimentation experiments. a, A representative SDS-PAGE co-sedimentation gel, where S and P designate supernatant and pellet fractions. b, Relative molar content of SipAC to actin in the pellet fractions plotted as mean values ± SD from three technical replicates. c, An overview (left) and zoomed-in view (right) of the inter-strand space in the vicinity of SipAC-Arm2-binding site, showing overlap between Arm2 (magenta), phalloidin (green; PDBID: 6T1Y) and jasplakinolide (yellow; PDBID: 6T24). d, Light grey indicates density around actin residues that do not participate in SipA binding; light pink – density around residues that interact with SipA, but not with phalloidin or jasplakinolide; purple – density around residues that interact with both SipA and phalloidin/jasplakinolide; blue – density around residues that interact with phalloidin or jasplakinolide, but not with SipA. The C-terminal tail of SipA is in magenta. e, FA analysis of TRITC-phalloidin binding to actin. f, FI analysis of SiR-actin binding to actin. g, Normalized FI competition data for SiR-actin with SipAC or SipAC-Core. h, Raw FI data for SiR-actin binding to F-actin and competition with unlabeled SipAC. i, Normalized FA competition data for TRITC-phalloidin with SipAC or SipAC-Core. j, Raw FA data for TRITC-phalloidin binding to actin and competition with unlabeled SipAC show that only ~50% of TRITC-phalloidin is displaced by SipAC.