Abstract
Progressive supranuclear palsy (PSP) is a neurodegenerative disease characterized by the accumulation of aggregated tau proteins in astrocytes, neurons, and oligodendrocytes. We performed whole genome sequencing (WGS) and conducted association analysis for single nucleotide variants (SNVs), small insertions/deletions (indels), and structural variants (SVs) in a cohort of 1,718 individuals with PSP and 2,944 control subjects. Analysis of common SNVs and indels confirmed known genetic loci at MAPT, MOBP, STX6, SLCO1A2, DUSP10, and SP1, and also uncovered novel signals in APOE, FCHO1/MAP1S, KIF13A, TRIM24, TNXB, and ELOVL1. In contrast to Alzheimer’s disease (AD), we observed the APOE ε2 allele to be the risk allele and the ε4 allele to be protective, a pattern similar to the association pattern observed in age-related macular degeneration (AMD) but the opposite observed for Alzheimer’s disease (AD). Analysis of rare SNVs and indels identified significant association in ZNF592 and further gene network analysis identified a module of neuronal genes dysregulated in PSP. We also observed seven common SVs associated with PSP on the H1/H2 haplotype region (17q21.31) and in a few other loci: IGH, PCMT1, CYP2A13, and SMCP. Particularly, in the H1/H2 haplotype region, there is a burden of rare deletions and duplications (P = 6.73×10−3) in PSP. Through WGS, we significantly refine our understanding of the genetic basis of PSP, providing new targets for exploring disease mechanisms and therapeutic interventions.
Keywords: Progressive Supranuclear Palsy (PSP), Whole-Genome Sequencing (WGS), Genome-Wide Association Study (GWAS), Structural Variants (SVs), Apolipoprotein E (APOE)
Introduction
Progressive supranuclear palsy (PSP) is a neurodegenerative disease that is pathologically defined by the accumulation of aggregated tau protein in multiple cortical and subcortical regions, especially involving the basal ganglia, dentate nucleus of the cerebellum midbrain1. An isoform of tau harboring 4 repeats of microtubule-binding domain (4R-tau) is particularly prominent in these tau aggregates2. Clinical manifestations of PSP include a range of phenotypes, including the initially described and most common, PSP-Richardson syndrome that presents with multiple features, including postural instability, vertical supranuclear palsy, and frontal dementia. However, there are several other phenotypes, such as PSP-Parkinsonism, PSP-Frontotemporal dementia, PSP-freezing of gait, PSP-speech and language disturbances, etc3. Presentation of these phenotypes varies widely depending on the distribution and severity of the pathology4,5.
Currently, the most recognized genetic risk locus for PSP is at the H1/H2 haplotype region covering MAPT gene at chromosome 17q21.316, where individuals carrying the common H1 haplotype are more likely to develop PSP with an estimated odds ratio (OR) of 5.67. Previous studies usually ascribed the observed association in the H1/H2 haplotype to MAPT6,8,9. However, recent functional dissection of this region using multiple parallel reporter assays coupled to CRISPRi demonstrated multiple risk genes in the area in addition to MAPT, including KANSL1 and PLEKMHL110. Genome-wide association studies (GWASs) in PSP have identified common variants in STX6, EIF2AK3, MOBP, SLCO1A2, DUSP10, RUNX2, and LRRK2 with moderate effect size7,11–13. In addition, variants in TRIM11 were identified as a genetic modifier of the PSP phenotype when comparing PSP with Richardson syndrome to PSP without Richardson syndrome14.
To date, no comprehensive analysis of single nucleotide variants (SNVs), small insertions and deletions (indels), and structural variants (SVs) in PSP by whole genome sequencing has been conducted. To gain a more comprehensive understanding of the genetic underpinnings of PSP, we performed whole genome sequencing (WGS) and analyzed SNVs, indels and SVs. As a result, we not only validated previously reported genes but also unveiled new loci that provide novel insights into the genetic basis of PSP.
Results
Common SNVs and indels associated with PSP
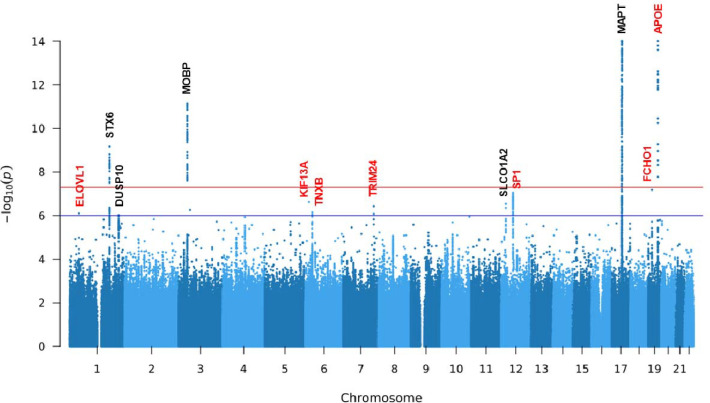
We conducted whole genome sequencing at 30x coverage (Methods) in 4,662 European-ancestry samples (1,718 individuals with PSP of which 1,441 were autopsy confirmed and 277 were clinically diagnosed and 2,944 control subjects, Table 1). We successfully replicated the association of known loci at MAPT, MOBP and STX67,11,12 and identified a novel signal in APOE with a genome-wide significance of P < 5 × 10−8 (Fig. 1, Fig. S1, Table 2, Table S1). Furthermore, eight loci showed suggestive significance (5 × 10 −8 < P < 1 × 10−6), including two loci reported genome-wide significant (SLCO1A2 and DUSP10) and one locus (SP1) reported suggestive significant in previous studies11,12, as well as five new loci in FCHO1/MAP1S, KIF13A, TRIM24, ELOVL1 and TNXB.
Table 1.
Characteristics of study participants.
| PSP (n = 1,718) | Control (n = 2,944) | ||
|---|---|---|---|
|
| |||
| Autopsy Confirmed (n = 1,441) | Clinical Diagnosed (n = 277) | ||
|
| |||
| Female | 625 (43%) | 129 (46%) | 1,775 (60%) |
|
| |||
| Age, y (SD) | 68.38 (8.22) | 65.72 (7.68) | 81.19 (6.01) |
|
| |||
| APOE ε4 a | |||
|
| |||
| ε4 carriers | 350 (24%) | 57 (21%) | 905 (32%) |
| Non-ε4 carriers | 1,085 (75%) | 216 (78%) | 1,913 (65%) |
| Data missing | 6 (0.42%) | 4 (1%) | 126 (4%) |
|
| |||
| APOE ε2b | |||
|
| |||
| ε2 carriers | 234 (16%) | 36 (13%) | 220 (8%) |
| Non-ε2 carriers | 1,193 (83%) | 238 (86%) | 2,522 (86%) |
| Data missing | 14 (1%) | 3 (1%) | 202 (7%) |
|
| |||
| H2 c | |||
|
| |||
| H2 carriers | 158 (11%) | 27 (10%) | 1,182 (40%) |
| Non-H2 carriers | 1,283 (89%) | 250 (90%) | 1,761 (60) |
| Data missing | 0 (0%) | 0 (0%) | 1 (0.03%) |
APOE ε4 is represented by the genotypes of rs429358-C.
APOE ε2 is represented by the genotypes of rs7412-T.
H2 haplotype is determined by the genotypes of rs8070723-G.
SD, standard deviation.
Fig. 1: Manhattan plot of SNVs/indels for PSP.
Loci with a suggestive or genome-wide significant signal are annotated (novel loci in red and known loci in black). Variants with a P- value below 1 × 10−14 are not shown. The red horizontal line represents genome-wide significance level (5 × 10−8). The blue horizontal line represents suggestive significance level (1 × 10−6).
Table 2.
Genome-wide and suggestive significant loci.
| SNV | Chr | Position | Ref | Alt | AF (Alt) | β (Alt) | P | Gene | eQTL/sQTL |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Genome-wide Significance (P < 5 × 10 −8) | |||||||||
|
| |||||||||
| rs62057121 | 17 | 45823394 | G | A | 0.15 | −1.32 | 7.45 × 10−78 | MAPT | LRRC37A4Pc* |
| rs4420638 | 19 | 44919689 | A | G | 0.20 | −0.57 | 2.91 × 10−19 | APOE | TOMM40b |
| rs7412 | 19 | 44908822 | C | T | 0.06 | 0.87 | 9.57 × 10−16 | APOE | |
| rs11708828 | 3 | 39458158 | C | T | 0.46 | −0.35 | 7.04 × 10−12 | MOBP | PRSAc |
| rs10753232 | 1 | 180980990 | C | T | 0.44 | 0.31 | 6.79 × 10−10 | STX6 | STX6a* |
|
| |||||||||
| Suggestive Significance (P < 1 × 10 −6) | |||||||||
|
| |||||||||
| rs56251816 | 19 | 17750888 | A | G | 0.22 | 0.35 | 6.57 × 10−08 | FCHO1/MAP1S | |
| rs12817984 | 12 | 53410523 | T | G | 0.16 | −0.37 | 8.91 × 10−08 | SP1 | SP1a* |
| rs4712314 | 6 | 17833813 | G | T | 0.51 | 0.27 | 2.37 × 10−07 | KIF13A | |
| rs74651308 | 12 | 21323155 | G | A | 0.07 | 0.51 | 2.86 × 10−07 | SLCO1A2 | |
| rs111593852 | 7 | 138449166 | C | T | 0.02 | 0.87 | 3.75 × 10−07 | TRIM24 | |
| rs367364 | 6 | 32052169 | C | T | 0.13 | −0.37 | 7.07 × 10−07 | TNXB | CYP21A1Pc* |
| rs839764 | 1 | 43367703 | T | A | 0.41 | 0.27 | 7.94 × 10−07 | ELOVL1 | TIE1a* |
| rs 1202665 9 | 1 | 221976623 | G | A | 0.21 | 0.31 | 9.48 × 10−07 | DUSP10 | |
Chr, chromosome; Ref, reference allele; Alt, alternative allele; AF, allele frequency.
Represents the SNV regulates multiple genes, and the gene with the smallest P-value was shown here (eQTL/sQTL for the brain region was obtained through GTEx).
SNVs with significant eQTL hits.
SNVs with significant sQTL hits.
SNVs with both eQTL and sQTL hits.
MAPT, MOBP and STX6
In the MAPT region, a multitude of SNVs and indels in high linkage disequilibrium (LD) with the H1/H2 haplotype remains the most significant association with PSP (Fig. S2A). From our analysis, the prominent signal within the MAPT region is rs62057121 (P = 7.45 × 10−78, β = −1.32, MAF = 0.15). Fine mapping (Methods) suggests that rs242561 (P = 4.49 × 10−74, β = −1.23, MAF = 0.16) is likely to be a causal SNV underling the statistical significance. The SNP rs242561 is located in an enhancer region, containing an antioxidant response element that binds with NRF2/sMAF protein complex. The T allele of rs242561 showed a stronger binding affinity for NRF2/sMAF in ChIP-seq analysis, therefore inducing a significantly higher transactivation of the MAPT gene15. rs242561 and rs62057151 were both in high LD (r2 > 0.9) with H1/H2 (defined by the 238 bp deletion in MAPT intron 9) and represented the same association signal as the H1/H2. However, in previous studies16,17, the H1c tagging SNV (rs242557) inside the H1/H2 region was found to be significant when conditioning on H1/H2. We confirmed that rs242557 was genome-wide significant after adjusting for H1/H2 (P = 3.68 × 10−15, β = 0.39, MAF = 0.42) though in weak LD with H1/H2 (r2 = 0.14). To pinpoint the causal genes underlying the association in H1/H2 requires additional functional study. For example, Cooper et al.10 analyzed transcriptional regulatory activity of SNVs and suggested PLEKHM1 and KANSL1 were probable causal genes in H1/H2 besides MAPT. In MOBP (rs11708828, P = 7.04 × 10−12, β = −0.35, MAF = 0.46, Fig. S2B) and STX6 (rs10753232, P = 6.79 × 10−10, β = 0.31, MAF = 0.44, Fig. S2C), the associated variants were of high allele frequency and exhibited moderate effect size.
APOE and risk of PSP
One newly identified significant locus from our analysis is the well-known Alzheimer’s Disease (AD) risk gene, APOE. We observed a significant association between the APOE ε2 haplotype and an elevated risk of PSP (P = 9.57 × 10−16, β = 0.87, MAF = 0.06, Table 3, Fig. S3B). The APOE ε2 haplotype is encoded by rs429358-T and rs4712-T, which is considered a protective allele in AD. The increased risk of APOE ε2 in PSP has been previously reported in a Japanese cohort, albeit with a relatively small sample size18. Furthermore, Zhao et al.19 confirmed that APOE ε2 is linked to increased tau pathology in the brains of individuals with PSP and reported a higher frequency of homozygosity of APOE ε2 in PSP with an odds ratio of 4.41. Consistent with these findings, our dataset exhibited a higher frequency of homozygosity of rs7412-T in PSP, yielding an odds ratio of 3.91.
Table 3.
Allele Frequency of APOE ε4 SNV (rs429358) 230 and ε2 SNV (rs7412)
| Studies | rs429358 | rs7412 | ||
|---|---|---|---|---|
| AF (Case) | AF (Control) | AF (Case) | AF (Control) | |
| PSP WGS (This study) | 0.1279 | 0.1742 | 0.0844 | 0.0414 |
| PSP GWAS25 | 0.1159 | 0.1366 | 0.0826 | 0.0794 |
| 1000 Genomes Project26 | 0.1512 | 0.0771 | ||
| ExAC European (non-Finnish)27 | 0.2078 | 0.1060 | ||
| gnomAD V4 European (non-Finnish)28 | 0.1506 | 0.0783 | ||
| TOPMed Freeze 8 NFE (Non-Finnish European) | 0.1501 | 0.0752 | ||
| ADSP R3 Non-Hispanic White29 | 0.3139 (AD as cases) | 0.1803 | 0.0244 (AD as cases) | 0.0406 |
For APOE ε4 allele, contrary to its association with AD, we observed that rs429358-C exhibits a protective effect against PSP (P = 5.71 × 10−18, β = −0.60, MAF = 0.16, Table 3). The lead SNV demonstrating this protective association from our analysis is rs4420638 (P = 2.91 × 10−19, β = −0.57, MAF = 0.20, Fig. S3A), which is in LD (r2 = 0.74) with rs429358. In a previous PSP GWAS conducted by Hoglinger et al.7, another APOE ε4 tagging SNV (rs2075650, r2 = 0.52 with rs429358) was also found to be diminished (MAF_case = 0.11 and MAF_control = 0.15) in PSP, although not reaching significance (P = 1.28 × 10−5). Notably, in our analysis, rs2075650 reached genome-wide significance (P = 3.39 × 10−13, β = −0.51, MAF = 0.15). APOE ε4 or ε2 displayed an independent effect for PSP risk without a significant epistatic interaction with H1/H2 haplotype (P > 0.05) (Fig. S4).
Given that our dataset included external controls from ADSP collected for Alzheimer’s disease studies, there were a potential selection biases for APOE ε4 and ε2 in controls. To address this concern, we broke down the allele frequencies of APOE ε4 and ε2 by cohorts (Table S2) and indicated cohorts with potential selection bias. The association analysis excluding these cohorts (Methods) shows the ε2 SNV (rs7412, P = 1.23 × 10−12, β = 0.70, MAF = 0.06) remained genome-wide significant and ε4 SNV (rs429358, P = 0.02, β = −0.16, MAF = 0.14) was nominal significant (Table S3, Table S4).
Suggestive significant loci
Eight loci were suggestive of significance in our analysis of which three, SLCO1A2, DUSP10, and SP1, were previously reported11,12. In SLCO1A2, the lead SNV rs74651308 (P = 2.86 × 10−7, β = 0.51, MAF = 0.07, Fig. S5A) is intronic and in LD (r2 = 0.98) with missense SNV rs11568563 (P = 1.45 × 10−6, β = 0.47, MAF = 0.07), which was reported in a previous study11. About 250 kb upstream of DUSP10 lies the previously reported SNV rs668775811 (P = 3.36 × 10−6, β = 0.29, MAF = 0.21), which is in LD (r2 = 0.98) with the lead SNV rs12026659 in our analysis (P = 9.48 × 10−7, β = 0.31, MAF = 0.21, Fig. S5B). In SP1, the reported indel rs14712428612 (P = 4.39 × 10−7, β = −0.35, MAF = 0.16) is in LD (r2 = 0.995) with the lead SNV rs12817984 (P = 8.91 × 10−8, β = −0.37, MAF = 0.16, Fig. S5C). Notably, disruption of a transcriptional network centered on SP1 by causal variants has been implicated previously in PSP10.
Five newly discovered suggestive loci are in FCHO1/MAP1S, KIF13A, TRIM24, TNXB, and ELOVL1. Within FCHO1/MAP1S, the most significant signal (rs56251816, P = 6.57 × 10−8, β = 0.35, MAF = 0.22, Fig. S6A) is in the intron of FCHO1. rs56251816 is a significant expression quantitative trait locus (eQTL) for both FCHO1 and MAP1S (13 kb upstream of FCHO1) in the Genotype-Tissue Expression (GTEx) project20. MAP1S encodes a microtubule associated protein that is involved in microtubule bundle formation, aggregation of mitochondria and autophagy21, and therefore, is more relevant than FCHO1 regarding PSP. KIF13A, which encodes a microtubule-based motor protein was also suggestive of significance (rs4712314, P = 2.37 × 10−7, β = 0.27, AF = 0.51, Fig. S6B). The significance in genes involved in microtubule-based processes, such as MAPT, MAP1S and KIF13A, implicates the neuronal cytoskeleton as a convergent aspect of PSP etiology.
Other variants with suggestive association evidence include TRIM24 (rs111593852, P = 3.75 × 10−7, β = 0.87, MAF = 0.02, Fig. S7A). TRIM24 is involved in transcriptional initiation and shows differential expression in individuals with Parkinson disease22,23. Another suggestive locus is TNXB, located in the major histocompatibility complex (MHC) region on chromosome 6, with the lead SNV rs367364 (P = 7.07 × 10−7, β = −0.37, MAF = 0.13, Fig. S7B). Finally, ELOVL1 yields suggestive evidence of association (rs839764, P = 7.94 × 10−7, β = 0.27, MAF = 0.41, Fig. S7C). This gene encodes an enzyme that elongates fatty acids and can cause a neurological disorder with ichthyotic keratoderma, spasticity, hypomyelination and dysmorphic features24. Furthermore, we found a few SNV/indels that reached genome-wide or suggestive significance without other supporting variants in LD (Fig.S1, Table S1). These signals could be due to sequencing errors and need further experimental validation.
Rare SNVs/indels and network analysis
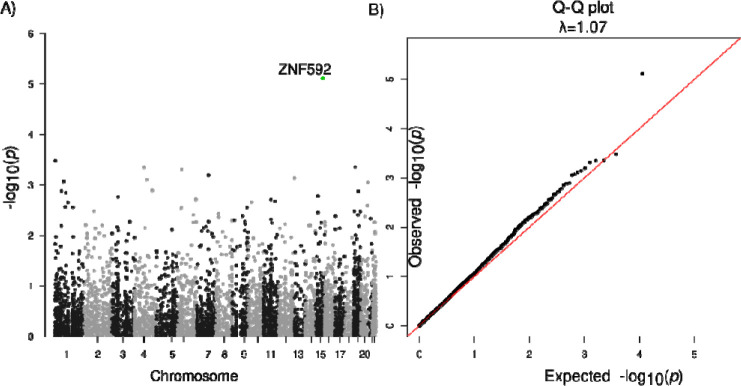
The heritability of PSP for common SNVs and indels (MAF > 0.01) was estimated to be 20%, while common plus rare SNVs/indels was estimated to be 23% from our analysis using GCTA-LDMS30 (Methods). Therefore, we performed aggregated tests for rare SNVs and indels, and identified ZNF592 (SKAT-O FDR=0.043, burden test FDR=0.041) with an of OR = 1.08 (95% CI: 1.008–1.16) (Fig. 2, Table 4, Table S5) for protein truncating or damaging missense variants (Methods). There was no genomic inflation with a λ=1.07 (Fig. 2). Risk in ZNF592 was imparted by 16 unique variants, with one splice donor and 15 damaging missense variants (Table S5). ZNF592 has not been previously associated with PSP but showed moderate RNA expression in the cerebellum compared to other tissues from GTEx (Fig. S8). There were no significant genes identified when evaluating protein-truncating variants (PTVs) only or when restricting to loss of function intolerant genes.
Fig. 2: Association analysis of rare SNVs/indels.
A. Manhattan plot for genes with protein truncating variants or damaging missense variants. B. Q-Q plot of gene P-values with protein truncating variants or damaging missense variants.
Table 4.
Association analysis of ZNF592 and the C1 module.
| Gene | Variants | Total MAC | Case MAC | Control MAC | Fraction Case | Fraction Control | OR (95% CI) | SKAT-O | Burden | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FDR | P | FDR | P | ||||||||
| ZNF592 | 16 | 19 | 8 | 11 | 0.0023 | 0.0018 | 1.08 (1.01–1.16) | 0.044 | 7.60×10−6 | 0.041 | 7.30×10−06 |
| Module | Variants | Total MAC | Case MAC | Control MAC | Fraction Case | Fraction Control | OR (95% CI) | Permutation test | P | Permutation test | P |
| C1 | 180 | 234 | 101 | 133 | 0.029 | 0.022 | 1.31 (1.01–1.70) | 0.19 | 0.048 | 0.078 | 0.006 |
Considering that genes do not operate along, but rather within signaling pathways and networks, we and others have shown that better understanding of disease mechanisms can be achieved through gene network analysis31–33. Therefore, we scrutinized rare variants within a network framework, focusing on co-expression network analysis performed in PSP post mortem brain that had previously identified a brain co-expression module, C1, which was conserved at the protein interaction level and enriched for common variants in PSP34. We found this C1 neuronal module was significantly enriched with PSP rare variants (P = 0.006, OR [95% CI] = 1.31 [1.01–1.70], Table 4; Table S3). Genes from the C1 module were more likely to be loss of function intolerant compared to the background of all brain expressed genes (Methods) (Fig. S8). To ensure that this was association not spurious, we performed permutation testing using random gene modules of brain expressed genes with the same number of genes as C1. The C1 module remains significant (Permutation P = 0.078). Exploring GTEx, we found that C1 genes are highly expressed in brain tissues including the cerebellum, frontal cortex, and basal ganglia (Fig. S8), consistent with regions affected in this disorder.
SVs associated with PSP
Seven high-confident SVs achieved genome-wide significance with PSP (Table 5, Fig. S9), including three deletions tagging the H2 haplotype. The most significant signal is a 238 bp deletion in MAPT intron 9 (Fig. S10A, chr17:46009357–46009595, P = 3.14 × 10−50, AF = 0.16) that has been reported on the H2 haplotype35,36 and is in LD (r2 = 0.99) with the lead SNV, rs62057121 (chr17:45823394, P = 7.45 × 10−78, β = −1.32, MAF = 0.15), in the MAPT region. Adding to this, two other deletions, one spanning 314 bp (Fig. S10B, chr17:46146541–46146855, AF = 0.19) and the other covering 323 bp (Fig. S10C, chr17:46099028–46099351, AF = 0.22), both are Alu elements and in LD (r2 > 0.8) with the top signal (the 238 bp deletion). This observation indicates that transposable elements may play an important role in the evolution of H1/H2 haplotype structure.
Table 5.
Significant structural variants from association analysis (P < 5 × 10−8).
| Name | N | AF | beta | P | AF (case) | AF (control) | Odds Ratio | Fisher’s P | Gene |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| chrl 7:46009357–46009595 :DEL* | 4357 | 0.16 | −1.22 | 3.14×10−50 | 0.054 | 0.23 | 0.19 | 5.80×10−118 | MAPT |
| chrl 7:46146541–46146855 :DEL* | 3697 | 0.19 | −1.12 | 2.13×10−39 | 0.079 | 0.25 | 0.26 | 1.58×10−83 | KANSL1 |
| chrl 7:46099028–46099351 :DEL* | 3699 | 0.22 | −1.07 | 3.88×10−37 | 0.11 | 0.28 | 0.33 | 2.05×10−66 | KANSL1 |
| chr14:105864208–105916743:DEL | 4378 | 0.010 | −1.53 | 4.74×10−14 | 0.0053 | 0.014 | 0.39 | 1.33×10−04 | IGH |
| chr6:149762615–149763234:DEL | 3811 | 0.55 | 0.50 | 8.60×10−12 | 0.75 | 0.42 | 4.19 | 6.00×10−182 | PCMT1 |
| chr19:41102802–41104285 :DEL | 2921 | 0.17 | 0.64 | 7.46×10−09 | 0.21 | 0.14 | 1.59 | 5.95×10−11 | CYP2A13 |
| chr1:152880979–152880979:INS | 2872 | 0.74 | 0.67 | 2.37×10−08 | 0.79 | 0.71 | 1.62 | 1.46×10−13 | SMCP |
Represents SVs with DNA samples available and PCR validated
Beyond the identified SVs in the H1/H2 region, we uncovered a significant deletion (chr14:105864208–105916743, P = 4.74 × 10−14, AF = 0.01) within the immunoglobulin heavy locus (IGH), which is a complex SV region (Fig. S11) related to antigen recognition. Moreover, a 619 bp deletion (chr6:149762615–149763234, P = 8.60 × 10−12, AF = 0.55; Fig. S10D) in PCMT1 displayed increased risk of PSP with an odds ratio of 4.19. The odds ratio increased to 8.38 when comparing 1,244 individuals with homozygous deletions in PCMT1 with the rest of sample set. PCMT1 encodes a type class of protein carboxyl methyltransferase enzyme that is highly expressed in the brain37 and is able to ameliorate Aβ25–35 induced neuronal apoptosis38,39. Additionally, we found a deletion between CYP2F1 and CYP2A13 (chr19:41102802–41104285, AF = 0.17) and an insertion in SMCP (chr1:152880979–152880979, AF = 0.74) which were also significant (Table 5). The 1.5 kb deletion (chr19:41102802–41104285) almost completely overlaps the SINE-VNTR-Alus (SVA) transposon region annotated by RepeatMasker40.
SVs in H1/H2 haplotype region
The H1/H2 region stands out as the pivotal genetic risk factor for PSP17,41. The H2 haplotype exhibits a reduced odds ratio of 0.19, as we observed the allele frequency of the 238 bp H2-tagging deletion is 23% in PSP and only 5% in control (P < 2.2 × 10−16). Moreover, our analysis pointed out five common (MAF > 0.01) and 12 rare deletions and duplications in the region (Table 6), ranging from 88 bp to 47 kb. Additionally, one common and four rare high-confidence insertions were reported in the region.
Table 6.
High-confident structural variants in the H1/H2 haplotype region
| Name | Size | N | AF | AF (PSP) | AF (Control) | Gene | Annotation |
|---|---|---|---|---|---|---|---|
|
| |||||||
| chrl 7:46099028–46099351 :DELa* | 323 | 3,699 | 0.24 | 0.11 | 0.28 | KANSL1 | intron |
| chr17:46146541–46146855:DELa* | 314 | 3,697 | 0.21 | 0.08 | 0.25 | KANSL1 | intron |
| chrl 7:46237619–46238142: DELa | 523 | 3,686 | 0.19 | 0.09 | 0.22 | MAPK8IP1P1 | intergenic |
| chr17:46009357–46009595:DELa* | 238 | 4,357 | 0.19 | 0.05 | 0.23 | MAPT | intron |
| chr17:46277789–46282210:DEL | 4,421 | 4,233 | 0.12 | 0.03 | 0.15 | ARL17B | intron |
|
| |||||||
| chr17:46113802–46113802:INS | 311 | 2,464 | 0.31 | 0.32 | 0.32 | KANSL1 | intron |
|
| |||||||
| Name | Size | N | N (Carriers) | N (PSP) | N (Control) | Gene | Annotation |
|
| |||||||
| chr17:46811121–46811289:DELa | 168 | 2,614 | 36 | 15 | 21 | WNT3 | intron |
| chr17:45847702–45851880:DELa | 4,178 | 4,427 | 31 | 17 | 14 | MAPT-AS1 | splicing |
| chr17:46837153–46839088:DELa | 1,935 | 4,415 | 12 | 8 | 4 | WNT9B | intron |
| chr17:45918825–45920861:DELa | 2,036 | 4,422 | 1 | 0 | 1 | MAPT | intron |
| chr17: 45 916681 –45920693 :DEL | 4,012 | 4,430 | 3 | 0 | 3 | MAPT | intron |
| chr17:45570198–45572012:DEL | 1,814 | 4,243 | 3 | 2 | 1 | AC091132.4 | intron |
| chr17:45334194–45381549:DELa | 47,355 | 4,430 | 1 | 0 | 1 | AC003070.2 | transcript ablation |
| chr17:45311955–45312258:DEL | 303 | 4,365 | 2 | 0 | 2 | MAP3K14 | intron |
| chr17:45894637–45914976:DUPa | 20,339 | 4,260 | 1 | 1 | 0 | MAPT-AS1 | transcript amplification |
| chr17:45993882–45993970:DELa | 88 | 4,283 | 1 | 1 | 0 | MAPT | splicing |
| chr17:45665996–45666370:DELa | 374 | 4,412 | 1 | 1 | 0 | LINC02210-CRHR1 | TFBS ablation |
| chr17:45879141–45881180:DEL | 2,039 | 4,431 | 1 | 1 | 0 | MAPT-AS1 | intron |
|
| |||||||
| chr17:45741582–45741582:INS | 315 | 4,420 | 10 | 4 | 6 | LINC02210-CRHR1 | intergenic |
| chr17:45929579–45929579:INS | 453 | 3,025 | 5 | 1 | 4 | MAPT | intron |
| chr17:46754483–46754483 :INS | 330 | 3,692 | 12 | 2 | 10 | NSF | intron |
AF, allele frequency; N, number of individuals with non-missing genotypes.
High-quality SVs that were included in association analysis.
Represents SVs with DNA samples available and PCR validated.
Of the five common deletions and duplications (Fig. S12), three show genome-wide significant association with the disease (Table 2); four are located in regions with transposable elements (SVA, L1, or Alu) and in LD (r2 from 0.63 to 0.92) with the 238 bp H2-tagging deletion (Methods). This further highlights the important role of transposable elements in shaping the landscape of H1/H2 region.
Among the 12 rare deletions and duplications (Fig. S13), five are located in potentially functional regions, such as splice sites, exons, and transcription factor binding sites (Table 6). Particularly, one deletion (chr17:45993882–45993970) in exon 9 of MAPT was identified in a PSP patient, adding to previous reports of exonic deletions in the MAPT in frontotemporal dementia, such as deletion of exon 1042 and exons 6–943 in MAPT. Using the SKAT-O test (N = 4,432), the 12 rare CNVs displayed a significantly higher burden in PSP than controls (P = 0.01, OR = 1.64).–
Discussion
Through comprehensive analysis of whole genome sequence, we identified SNVs, indels and SVs contributing to the risk of PSP. For common SNVs, previously reported regions, including MAPT, MOBP, STX6, SLCO1A2, DUSP10, and SP17,11,12 were replicated in our analysis and novel loci in APOE, FCHO1/MAP1S, KIF13A, TRIM24, ELOVL1, and TNXB were discovered. EIF2AK3 which was significantly associated with PSP in a previous GWAS17 did not reach significance in our study. The SNV with the lowest P around EIF2AK3 was rs13003510 (P = 8.30 × 10−5, β = 0.22, MAF = 0.3).
The APOE ε4 haplotype was of particular interest as it is a common risk factor for AD, explaining more than a 1/3 of population attributable risk44,45. Typically, individuals with one copy of the APOE ε4 allele (rs429358-C and rs4712-G) have approximately a threefold increased risk of developing AD, while those with two copies of the allele have an approximately a 12-fold increase in risk46. In striking contrast, the ε4 tagging allele rs429358 was protective in PSP and the ε2 tagging allele rs7412 was deleterious. This observation is particularly intriguing since both AD and PSP have intracellular aggregated tau as a prominent neuropathologic feature. Notably, both ε2 allele and ε4 allele have been associated with tau pathology burden in the brain of mice models19,47, which raises the question of distinct tau species in 4R-PSP versus 3R-4R-AD. It is also notable that the ε2 allele is also associated with increased risk for age-related macular degeneration (AMD), and the ε4 allele was associated with decreased risk48,49. These results demonstrate that the same variant may have opposite effects in different degenerative diseases. This is especially important, given the advent of gene editing as a therapeutic modality, and programs focused on changing APOE ε4 to ε2. Although this therapy would likely decrease risk for AD, our results indicate that it would increase risk for PSP, in addition to AMD. From this standpoint, caution is warranted in germ-line genome editing until the broad spectrum of phenotypes associated with human genetic variation is understood.
Burden association tests are an highly valuable for addressing sample size limitations in analyzing rare variats50. Indeed, burden testing allowed us to identify ZNF592, a classical C2H2 zinc finger protein (ZNF)51,52, as a candidate risk gene. ZNF proteins have been causative or strongly associated with large numbers of neurodevelopmental disease53,54 and neurodegenerative disease including Parkinson’s disease55 and Alzheimer’s disease56,57. ZNF592 was initially thought to be responsible for autosomal recessive spinocerebellar ataxia 5 from a consanguineous family with neurodevelopmental delay including cerebellar ataxia and intellectual disability due to a homozygous G1046R substitution58. However, further analysis of this family identified WDR73 to be the most likely causative gene, consistent with Galloway-Mowat syndrome, although ZNF592 may have contributed to the phenotype59.
We also extended classical gene-based burden analysis to consider rare risk burden in the context of a gene set defined by co-expression networks34,60. We leveraged combined previous proteomic and transcriptomic analysis of post-mortem brain from patients afflicted with PSP, and showed that rare variants enrich in the C1 neuronal module, which was the same module enriched with common variants34. This, along with our recent work identifying a neuronally-enriched transcription factor network centered around SP1 disrupted by PSP common genetic risk, suggests that although PSP neuropathologically is defined by tufted astrocytes and oligodendroglial coiled bodies61–63, initial causal drivers of PSP appear to be primarily neuronal.
In analysis of SVs, we found deletions in PCMT1 and IGH were significantly associated with PSP. The IGH deletions are in a complex region on chromosome 14 that encodes immunoglobins recognizing foreign antigens. The size of the IGH deletion varies across individuals (Fig. S9). In addition, the IGH deletions can be accompanied by other deletions, duplications, and inversions (Fig. S9). These combined make the experimental validation of the deletion challenging. The PCMT1 deletion is common (AF = 0.55) with an odds ratio of 8.38 for PSP in homozygous individuals.
There were limitations to this study. Not all PSP were pathologically confirmed, although pathological confirmation was available in a significant subset (of the 1,718 PSP individuals, 1,441 were autopsy-confirmed and 277 were clinically-diagnosed). Additionally, the majority of control samples in this study were from ADSP and were initially collected as controls for AD studies. As ADSP is a dataset composed of multiple cohorts from diverse sources, it is imperative to ensure that any observed allele frequency differences between controls and cases can be attributed to the disease itself rather than sample selection biases arising from technical artifacts or batch effects. To mitigate the risk of false reports, we meticulously examined the allele frequencies of both cases and controls, especially in relation to novel and significant signals.
This work represents an important first step; future work is necessary to further delineate the rare genetic risk in PSP harbored in coding and noncoding regions. These results may come to fruition as additional genomic analytical methods are developed, sample size increased, and orthogonal genomic data are integrated. While PSP is rare, it is the most common primary tauopathy, and studying this disease is critical to understanding common pathological mechanisms across tauopathies. Further work to include individuals with diverse ancestry background will also improve our understanding of genetic architecture of the disease.
Methods
Study subjects
We performed WGS at 30x coverage (Table S7) for 1,834 PSP cases and 128 controls from the PSP-NIH-CurePSP-Tau, PSP-CurePSP-Tau, PSP-UCLA, and AMPAD-MAYO cohorts included in ADSP (ng00067) and used 3,008 controls from ADSP64. Control subjects were self-identified as non-Hispanic white. WGS data is available on NIAGADS65. We removed related subjects (IBD>0.25), five clinically diagnosed PSP who were not found to have PSP on autopsy, and non-Europeans (subjects that were eight standard deviations away from the 1000 Genomes Project European samples26,66 using the first six principal components), resulting in 1,718 individuals with PSP and 2,944 control subjects. Of the 1,718 PSP individuals, 1,441 were autopsy-confirmed and 277 were clinically-diagnosed (Table 1).
Given that our dataset included external controls from ADSP collected for Alzheimer’s disease studies, there was a potential selection biases for APOE ε4 and ε2 in controls. We broke down the allele frequencies of APOE ε4 and ε2 by cohorts (Table S2) and reviewed the study design of each cohort. The ADSP-FUS1-APOEextremes study used an age extremes sampling approach stratified by APOE genotype, comparing younger onset AD cases against older cognitively normal controls: the controls were APOE ε4/ε4 controls with age-at-last-assessment ≥ 75 years, APOE ε3/ε4 controls with age-at-last-assessment ≥ 80 years, or APOE ε3/ε3 controls with age-at-last-assessment ≥ 85 years64. The ADSP-FUS1-StEPAD1 study aims to identify and characterize novel genetic variants that promote resilience to AD pathology in the presence of the APOE4 allele: controls from ADSP-FUS1-StEPAD1 were protected APOE4 carriers have normal cognition at older age64. The CacheCounty study selects “AD resilient individuals” and define them as individuals who are at least 75 years old, cognitively normal, and carry at least one APOE ε4 allele67.
Common SNVs/indels analysis
Only biallelic variants were included in common SNVs/indels analysis. Variants were removed if they were monomorphic, did not pass variant quality score recalibration (VQSR), had an average read depth ≥ 500, or if all calls have DP<10 & GQ<20. Individual calls with a DP<10 or GQ<20 were set to missing. Indels were left aligned using the GRCh38 reference68,69. Common variants (MAF > 0.01) with a missing rate < 0.1, 0.25 < ABHet < 0.75 and HWE (in control) > 1 × 10−5 were kept for analysis, leaving 7,945,112 SNVs/indels for analysis. Genetic relatedness matrix was obtained using KING70. Principal components were obtained by PC-AiR71 which accounts for sample relatedness. Linear mixed model implemented in R Genesis72 were used for association. Sex and PC1–5 were adjusted in the linear mixed model. Age was not adjusted as more than half (1,159 of 1,718) of PSP cases had age missing. After association, variants with a P < 1 × 10−6 were reported. For SNVs/indels without supporting evidence from nearby SNVs/indels in LD, we removed possible spurious calls with FS (Phred-scaled P-value using Fisher’s exact test to detect strand bias) > 4, VQSLOD (Log odds of being a true variant versus being false under the trained gaussian mixture model) < 15, or located in regions of genome showing discrepancy from Telomere-to-Telomere Consortium73 and GRCh38. Fine-mapping of the H1/H2 region were analyzed using SuSie74. We ran the analysis several times assuming the number of maximum causal variants were from 2 to 10. The only variant (rs242561) robust to the choice of maximum causal variants was reported. To avoid potential confounding effects (particularly for APOE alleles), we also performed association analysis (Table S4) for suggestive and genome-wide significant signals when excluding subjects from the three cohorts with selection bias against APOE alleles along with cohorts with less than 10 subjects (NACC-Genentech, FASe-Families-WGS, KnightADRC-WGS).
Rare SNVs/indels analysis
Multi-allelic variants were split into biallelic variants. Variants where ALT=*, representing a spanning deletion, were removed. Biallelic and multiallelic variants were concatenated, and duplicated variants were removed. Variants were removed if they were monomorphic, did not pass VQSR, had an average read depth ≥ 500, or if all calls have DP<10 & GQ<20. Individual calls with a DP<10 or GQ<20 were set to missing. Indels were left aligned using the GRCh38 reference68,69. Then, variants with a missing rate > 0.1 or a PHWE < 1 × 10−7 in controls were removed, resulting in 91,863,622 variants. We calculated the heritability of PSP using GCTA-LDMS30 for common SNVs/indels (MAF > 0.01) and common plus rare SNVs/indels. A prevalence of 5 PSP cases per 100,000 individuals (0.00005) was used in the GCTA-LDMS analysis.
For aggregated tests of rare variants, we considered rare protein truncating variants (PTVs) and PTVs/damaging missense variants. Variant were annotated with ANNOVAR (version 2020–06-07)75 and Variant Effect Predictor (VEP, version 104.3)76. PTVs were in protein coding genes (Ensembl,version 104)77 and had VEP consequence as stop gained, splice acceptor, splice donor or frameshift. Damaging missense variants were in protein coding genes (Ensembl version 104)77 and had a VEP consequence as missense, CADD score ≥ 15, and PolyPhen-2 HDIV of probably damaging. Rare variants were selected based on a MAF < 0.01% from gnomAD and a MAF < 1% in our dataset. The number of alternative allele variants in protein truncating variants (PTV) and PTV/damaging missense variants was similar across sequencing centers and when evaluated for loss of function intolerant genes (observed/expected score upper confidence interval < 0.3578) (Fig. S14)
After LD clumping with a r2 cutoff of 0.2, we applied the bigsnpr R package to perform PCA using variants with MAF > 1%. We tested if genes with PTVs or PTVs/missense variants were associated with PSP using the sequence kernel association test-optimized (SKAT-O)79 (SKAT R package version 2.0.1)80. We used a linear kernel and weighed each variant by the maximum external database MAF where lower MAF would have higher weight. We normalized variant MAFs, where , where is the external database MAF from gnomAD78 and . The variant weight is defined by the on the β (1,4) distribution. Thus, variant weight is high at very low and spread across the range of 1 to 4. Covariates included sex, PC1–3, and H1/H2 haplotype. P-values were FDR corrected for the number of genes with a total minor allele count (MAC) ≥ 10. In addition to SKAT-O, we performed gene burden testing (SKATBinary method=‘burden’). As SKAT-O does not calculate an odds ratio, we calculated the odds ratio of significant genes using logistic regression with the same covariates as SKAT-O and burden testing, and the same variant weights. We also considered only PTVs or PTVs/missense variants in loss of function intolerant genes (observed/expected score upper confidence interval < 0.3578).
We evaluated the C1 module, a gene set, which was previously shown to be composed of neuronal genes and enriched for common variants in PSP34. We performed a permutation test (N=1000) of random gene set modules from brain expressed genes that contained the same number of genes as C1. From the human protein atlas (www.proteinatlas.org)81, brain expressed genes were defined as the union of unique proteins from the cerebral cortex, basal ganglia and midbrain (N=15,638). We calculated SKAT-O P-values from these random gene modules to determine the null distribution. We calculated the unadjusted odds ratio of significant genes or gene sets by summing the number of alternate alleles in the gene set among the total number alleles in cases and controls。
Normalized quantification (TPM) gene expression across tissues was obtained from Genotype-Tissue Expression (GTEx)82. The expression of ZNF592 and C1 module (summarized as an eigengene83) were plotted.
SV detection and filtering
For each sample, SVs were called by Manta84 (v1.6.0) and Smoove85 (v0.2.5) with default parameters. Calls from Manta and Smoove were merged by Svimmer 86 to generate a union of two call sets for a sample. Then, all individual sample VCF files were merged together by Svimmer as input to Graphtyper2 (v2.7.3)86 for joint genotyping. SV calls after joint-genotyping are comparable across the samples, therefore, can be used directly in genome-wide association analysis86. A subset of SV calls was defined as high-quality calls86. Details of SV calling pipeline were in our previous study87.
There are regions in the human genome that tend to have anomalous, or high signal in WGS experiments88. SVs that reside in those regions can be unreliable and should be reported. Specifically, we compiled problematic regions in the genome from the following sources: (1) the ENCODE blacklist: a comprehensive set of regions that could result in erroneous signal89; (2) the 1000 Genome masks: regions of the genome that are more or less accessible to next generation sequencing methods using short reads; (3) the set of assembly gaps defined by UCSC; (4) the set of segmental duplications defined by UCUC; (5) the low-complexity regions, satellite sequences and simple repeats defined by RepeatMasker40. For each individual SV reported, Samplot90 or IGV91 were used to keep only high-confident CNVs and inversions that are supported by read depth or split reads; for insertions, we kept high-confident insertions that are high-quality and not in the masked regions.
SV analysis
For SV association, more strict sample filtering was applied: outlier samples with too many (larger than median + 4*MAD) CNV/insertion calls or too little (smaller than median - 4*MAD) high-quality CNV/insertion calls were removed. There were 4,432 samples (1,703 cases and 2,729 controls) remaining for PSP SV association analysis. Due to more false positives being picked up, the genomic inflation would be high (λ = 1.89, Fig. S9) if all SVs were included in the analysis. Therefore, we restricted our analysis to high-quality SVs only, making the genomic inflation drop to 1.27 (Fig. S9). The 14,792 high-quality common SVs (MAF > 0.1) with call rate > 0.5 were included in the analysis. Mixed model implemented in R Genesis72 were used for association. Sex, PCR information, SV PCs 1–5, and SNV PCs 1–5 were adjusted in the mixed model. After association, we manually inspect deletions, duplications, and inversions by Samplot90 or IGV91 to keep only those with support from read depth, split read or insert size. For insertions, those not on masked regions were reported.
For SVs inside the H1/H2 region, all SVs those that are not high-quality are included. Then, we removed SVs with missing rate > 0.5 and manual inspect deletions, duplications, and inversions by Samplot90 or IGV91 to keep only those with support from read depth, split read or insert size. For insertions, those high-quality ones not on masked regions were kept for analysis. LD between SVs was calculated using PLINK (V1.90 beta)92.Rare SV burden on H1/H2 region was evaluated by SKAT-O79 adjusting for gender and PCs 1–5. As SKAT-O does not calculate an odds ratio, we calculated the odds ratio using logistic regression with the same covariates.
Supplementary Material
Acknowledgements
This project is supported by CurePSP, courtesy of a donation from the Morton and Marcine Friedman Foundation. We are indebted to the Biobanc-Hospital Clinic-FRCB-IDIBAPS for samples and data procurement. The acknowledgement of PSP cohorts is listed below, whereas the acknowledgement of ADSP cohorts for control samples can be found in the supplementary materials. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The data used for the analyses described in this manuscript were obtained from: https://gtexportal.org/home/datasets the GTEx Portal on 1/27/2022. We also thank to Drs. Murray Grossman and Hans Kretzschmar for their valuable contribution to this work.
AMP-AD (sa000011) data: Mayo RNAseq Study- Study data were provided by the following sources: The Mayo Clinic Alzheimer’s Disease Genetic Studies, led by Dr. Nilufer Ertekin-Taner and Dr. Steven G. Younkin, Mayo Clinic, Jacksonville, FL using samples from the Mayo Clinic Study of Aging, the Mayo Clinic Alzheimer’s Disease Research Center, and the Mayo Clinic Brain Bank. Data collection was supported through funding by NIA grants P50 AG016574, R01 AG032990, U01 AG046139, R01 AG018023, U01 AG006576, U01 AG006786, R01 AG025711, R01 AG017216, R01 AG003949, NINDS grant R01 NS080820, CurePSP Foundation, and support from Mayo Foundation. Study data includes samples collected through the Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona. The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05–901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
PSP-NIH-CurePSP-Tau (sa000015) data: This project was funded by the NIH grant UG3NS104095 and supported by grants U54NS100693 and U54AG052427. Queen Square Brain Bank is supported by the Reta Lila Weston Institute for Neurological Studies and the Medical Research Council UK. The Mayo Clinic Florida had support from a Morris K. Udall Parkinson’s Disease Research Center of Excellence (NINDS P50 #NS072187), CurePSP and the Tau Consortium. The samples from the University of Pennsylvania are supported by NIA grant P01AG017586.
PSP-CurePSP-Tau (sa000016) data: This project was funded by the Tau Consortium, Rainwater Charitable Foundation, and CurePSP. It was also supported by NINDS grant U54NS100693 and NIA grants U54NS100693 and U54AG052427. Queen Square Brain Bank is supported by the Reta Lila Weston Institute for Neurological Studies and the Medical Research Council UK. The Mayo Clinic Florida had support from a Morris K. Udall Parkinson’s Disease Research Center of Excellence (NINDS P50 #NS072187), CurePSP and the Tau Consortium. The samples from the University of Pennsylvania are supported by NIA grant P01AG017586. Tissues were received from the Victorian Brain Bank, supported by The Florey Institute of Neuroscience and Mental Health, The Alfred and the Victorian Forensic Institute of Medicine and funded in part by Parkinson’s Victoria and MND Victoria. We are grateful to the Sun Health Research Institute Brain and Body Donation Program of Sun City, Arizona for the provision of human biological materials (or specific description, e.g. brain tissue, cerebrospinal fluid). The Brain and Body Donation Program is supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services ( contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05–901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research. Biomaterial was provided by the Study Group DESCRIBE of theClinical Research of the German Center for Neurodegenerative Diseases (DZNE).
PSP_UCLA (sa000017) data: Thank to the AL-108–231 investigators, Adam L Boxer, Anthony E Lang, Murray Grossman, David S Knopman, Bruce L Miller, Lon S Schneider, Rachelle S Doody, Andrew Lees, Lawrence I Golbe, David R Williams, Jean-Cristophe Corvol, Albert Ludolph, David Burn, Stefan Lorenzl, Irene Litvan, Erik D Roberson, Günter U Höglinger, Mary Koestler, Cliff ord R Jack Jr, Viviana Van Deerlin, Christopher Randolph, Iryna V Lobach, Hilary W Heuer, Illana Gozes, Lesley Parker, Steve Whitaker, Joe Hirman, Alistair J Stewart, Michael Gold, and Bruce H Morimoto.
Funding
This work was supported by NIH 5UG3NS104095, the Rainwater Charitable Foundation, and CurePSP. HW and PLC are supported by RF1-AG074328, P30-AG072979, U54-AG052427 and U24-AG041689. TSC is supported by NIH K08AG065519 and the Larry L Hillblom Foundation 2021-A-005-SUP. KF was supported by CurePSP 685-2023-06-Pathway and K01 AG070326. MG is supported by P30 AG066511. BFG and KLN are supported by P30 AG072976 and R01 AG080001. TGB and GES are supported by P30AG072980. IR is supported by 2R01AG038791-06A, U01NS100610, R25NS098999, U19 AG063911-1 and 1R21NS114764-01A1. OR is support by U54 NS100693. DG is supported by P30AG062429. ALB is supported by U19AG063911, R01AG073482, R01AG038791, and R01AG071756. BLM is supported by P01 AG019724, R01 AG057234 and P0544014. VMV is supported by P01-AG-066597, P01-AG-017586. HRM is supported by CurePSP, PSPA, MRC, and Michael J Fox Foundation. RDS is supported by CurePSP, PSPA, and Reta Lila Weston Trust. JFC is supported by R01 AG054008, R01 NS095252, R01 AG060961, R01 NS086736, R01 AG062348, P30 AG066514, the Rainwater Charitable Foundation / Tau Consortium, Karen Strauss Cook Research, and Scholar Award, Stuart Katz & Dr. Jane Martin. AMG is supported by the Tau Consortium and U54-NS123746. YYL is supported by U54-AG052427; U24-AG041689. LSW is supported by U01AG032984, U54AG052427, and U24AG041689. DHG is supported by 3UH3NS104095, Tau Consortium. WPL is supported by RF1-AG074328; P30-AG072979; U54-AG052427; U24-AG041689. Cases from Banner Sun Health Research Institute were supported by the NIH (U24 NS072026, P30 AG19610 and P30AG072980), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research. The Mayo Clinic Brain Bank is supported through funding by NIA grants P50 AG016574, CurePSP Foundation, and support from Mayo Foundation.
Footnotes
Declarations
Competing interests
Laura Molina-Porcel received income from Biogen as a consultant in 2022. Gesine Respondek is employed by Roche (Hoffmann-La Roche, Basel, Switzerland) since 2021. Her affiliation whilst completing her contribution to this manuscript was München Technische Universität München, German Center for Neurodegenerative Diseases (DZNE), Munich. Thomas G Beach is a consultant for Aprinoia Therapeutics and a Scientific Advisor and stock option holder for Vivid Genomics. Huw Morris is employed by UCL. In the last 12 months he reports paid consultancy from Roche, Aprinoia, AI Therapeutics and Amylyx; lecture fees/honoraria - BMJ, Kyowa Kirin, Movement Disorders Society. Huw Morris is a co-applicant on a patent application related to C9ORF72 - Method for diagnosing a neurodegenerative disease (PCT/GB2012/052140). Giovanni Coppola is currently an employee of Regeneron Pharmaceuticals. Alison Goate serves on the SAB for Genentech and Muna Therapeutics.
Ethics approval and consent to participate
Consent for publication
Not applicable.
Availability of data and materials
NIAGADS Data Sharing Service (https://dss.niagads.org/)
https://github.com/whtop/PSP-Whole-Genome-Sequencing-Analysis
References
- 1.Hauw J.-J. et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology 44, 2015–2015 (1994). [DOI] [PubMed] [Google Scholar]
- 2.Stamelou M. et al. Evolving concepts in progressive supranuclear palsy and other 4-repeat tauopathies. Nat. Rev. Neurol. 17, 601–620 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Hoglinger G. U. et al. Clinical Diagnosis of Progressive Supranuclear Palsy: The Movement Disorder Society Criteria. Mov. Disord. Off. J. Mov. Disord. Soc. 32, 853–864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lukic M. J. et al. Long-Duration Progressive Supranuclear Palsy: Clinical Course and Pathological Underpinnings. Ann. Neurol. 92, 637–649 (2022). [DOI] [PubMed] [Google Scholar]
- 5.Ali F. et al. Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy. Mov. Disord. 34, 1144–1153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wen Y., Zhou Y., Jiao B. & Shen L. Genetics of progressive supranuclear palsy: a review. J. Park. Dis. 11, 93–105 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Höglinger G. U. et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 43, 699–705 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borroni B., Agosti C., Magnani E., Di Luca M. & Padovani A. Genetic bases of Progressive Supranuclear Palsy: the MAPT tau disease. Curr. Med. Chem. 18, 2655–2660 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Rademakers R., Cruts M. & Van Broeckhoven C. The role of tau (MAPT) in frontotemporal dementia and related tauopathies. Hum. Mutat. 24, 277–295 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Cooper Y. A. et al. Functional regulatory variants implicate distinct transcriptional networks in dementia. Science 377, eabi8654 (2022). [DOI] [PubMed] [Google Scholar]
- 11.Sanchez-Contreras M. Y. et al. Replication of progressive supranuclear palsy genome-wide association study identifies SLCO1A2 and DUSP10 as new susceptibility loci. Mol. Neurodegener. 13, 1–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J. A. et al. Joint genome-wide association study of progressive supranuclear palsy identifies novel susceptibility loci and genetic correlation to neurodegenerative diseases. Mol. Neurodegener. 13, 1–11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabbari E. et al. Genetic determinants of survival in progressive supranuclear palsy: a genome-wide association study. Lancet Neurol. 20, 107–116 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabbari E. et al. Variation at the TRIM11 locus modifies progressive supranuclear palsy phenotype. Ann. Neurol. 84, 485–496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X. et al. A polymorphic antioxidant response element links NRF2/sMAF binding to enhanced MAPT expression and reduced risk of Parkinsonian disorders. Cell Rep. 15, 830–842 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anaya F., Lees A. & Silva R. Tau gene promoter rs242557 and allele-specific protein binding. Transl. Neurosci. 2, (2011). [Google Scholar]
- 17.Höglinger G. U. et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 43, 699–705 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawa A. et al. Apolipoprotein E in progressive supranuclear palsy in Japan. Mol. Psychiatry 2, 341–342 (1997). [DOI] [PubMed] [Google Scholar]
- 19.Zhao N. et al. APOE ε2 is associated with increased tau pathology in primary tauopathy. Nat. Commun. 9, 4388 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lonsdale J. et al. The genotype-tissue expression (GTEx) project. Nat. Genet. 45, 580 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie R. et al. Microtubule-associated protein 1S (MAP1S) bridges autophagic components with microtubules and mitochondria to affect autophagosomal biogenesis and degradation. J. Biol. Chem. 286, 10367–10377 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi L. et al. Pilot study: molecular risk factors for diagnosing sporadic Parkinson’s disease based on gene expression in blood in MPTP-induced rhesus monkeys. Oncotarget 8, 105606 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pan M. et al. Tripartite Motif Protein Family in Central Nervous System Diseases. Cell. Mol. Neurobiol. 1–23 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kutkowska-Kaźmierczak A. et al. Dominant ELOVL1 mutation causes neurological disorder with ichthyotic keratoderma, spasticity, hypomyelination and dysmorphic features. J. Med. Genet. 55, 408–414 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Farrell K. et al. Genetic, transcriptomic, histological, and biochemical analysis of progressive supranuclear palsy implicates glial activation and novel risk genes. bioRxiv 2023–11 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Consortium, 1000 Genomes Project. A global reference for human genetic variation. Nature vol. 526 68 (Nature Publishing Group, 2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lek M. et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S. et al. A genome-wide mutational constraint map quantified from variation in 76,156 human genomes. bioRxiv 2022–03 (2022). [Google Scholar]
- 29.Lee W.-P. et al. Association of Common and Rare Variants with Alzheimer’s Disease in over 13,000 Diverse Individuals with Whole-Genome Sequencing from the Alzheimer’s Disease Sequencing Project. medRxiv 2023–09 (2023). [Google Scholar]
- 30.Yang J. et al. Genetic variance estimation with imputed variants finds negligible missing heritability for human height and body mass index. Nat. Genet. 47, 1114–1120 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swarup V. et al. Identification of evolutionarily conserved gene networks mediating neurodegenerative dementia. Nat. Med. 25, 152–164 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swarup V. et al. Identification of conserved proteomic networks in neurodegenerative dementia. Cell Rep. 31, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parikshak N. N., Gandal M. J. & Geschwind D. H. Systems biology and gene networks in neurodevelopmental and neurodegenerative disorders. Nat. Rev. Genet. 16, 441–458 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swarup V. et al. Identification of Conserved Proteomic Networks in Neurodegenerative Dementia. Cell Rep. 31, 107807 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baker M. et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum. Mol. Genet. 8, 711–715 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Wang H., Wang L.-S., Schellenberg G. & Lee W.-P. The role of structural variations in Alzheimer’s disease and other neurodegenerative diseases. Front. Aging Neurosci. (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mizobuchi M., Murao K., Takeda R. & Kakimoto Y. Tissue-specific expression of isoaspartyl protein carboxyl methyltransferase gene in rat brain and testis. J. Neurochem. 62, 322–328 (1994). [DOI] [PubMed] [Google Scholar]
- 38.Wu X. et al. Neural stem cell-conditioned medium upregulated the PCMT1 expression and inhibited the phosphorylation of MST1 in SH-SY5Y cells induced by Aβ 25–35. Biocell 46, 471 (2022). [Google Scholar]
- 39.Shi L. et al. PCMT1 ameliorates neuronal apoptosis by inhibiting the activation of MST1 after subarachnoid hemorrhage in rats. Transl. Stroke Res. 8, 474–483 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Smit AFA, Hubley R & Green P. RepeatMasker Open-4.0. 2013-2015 <http://www.repeatmasker.org.>
- 41.Chen J. A. et al. Joint genome-wide association study of progressive supranuclear palsy identifies novel susceptibility loci and genetic correlation to neurodegenerative diseases. Mol. Neurodegener. 13, 41 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rizzu P. et al. High prevalence of mutations in the microtubule-associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am. J. Hum. Genet. 64, 414–421 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rovelet-Lecrux A. et al. Partial deletion of the MAPT gene: A novel mechanism of FTDP-17. Hum. Mutat. 30, E591–E602 (2009). [DOI] [PubMed] [Google Scholar]
- 44.Farrer L. A. et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. Jama 278, 1349–1356 (1997). [PubMed] [Google Scholar]
- 45.Selkoe D. J. & Podlisny M. B. Deciphering the genetic basis of Alzheimer’s disease. Annu. Rev. Genomics Hum. Genet. 3, 67–99 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Bertram L., McQueen M. B., Mullin K., Blacker D. & Tanzi R. E. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat. Genet. 39, 17–23 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Shi Y. et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 549, 523–527 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rasmussen K. L., Tybjærg-Hansen A., Nordestgaard B. G. & Frikke-Schmidt R. Associations of Alzheimer disease–protective APOE variants with age-related macular degeneration. JAMA Ophthalmol. 141, 13–21 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klaver C. C. et al. Genetic association of apolipoprotein E with age-related macular degeneration. Am. J. Hum. Genet. 63, 200–206 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S., Abecasis G. R., Boehnke M. & Lin X. Rare-variant association analysis: study designs and statistical tests. Am. J. Hum. Genet. 95, 5–23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cassandri M. et al. Zinc-finger proteins in health and disease. Cell Death Discov. 3, 1–12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fedotova A. A., Bonchuk A. N., Mogila V. A. & Georgiev P. G. C2H2 Zinc Finger Proteins: The Largest but Poorly Explored Family of Higher Eukaryotic Transcription Factors. Acta Naturae 9, 47–58 (2017). [PMC free article] [PubMed] [Google Scholar]
- 53.Bu S., Lv Y., Liu Y., Qiao S. & Wang H. Zinc Finger Proteins in Neuro-Related Diseases Progression. Front. Neurosci. 15, 760567 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Naama N., Mackeh R. & Kino T. C2H2-Type Zinc Finger Proteins in Brain Development, Neurodevelopmental, and Other Neuropsychiatric Disorders: Systematic Literature-Based Analysis. Front. Neurol. 11, 32 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin J.-H. et al. PARIS (ZNF746) Repression of PGC-1α Contributes to Neurodegeneration in Parkinson’s Disease. Cell 144, 689–702 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li R., Strohmeyer R., Liang Z., Lue L.-F. & Rogers J. CCAAT/enhancer binding protein delta (C/EBPdelta) expression and elevation in Alzheimer’s disease. Neurobiol. Aging 25, 991–999 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Ko C.-Y. et al. CCAAT/enhancer binding protein delta (CEBPD) elevating PTX3 expression inhibits macrophage-mediated phagocytosis of dying neuron cells. Neurobiol. Aging 33, 422.e11–25 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nicolas E. et al. CAMOS, a nonprogressive, autosomal recessive, congenital cerebellar ataxia, is caused by a mutant zinc-finger protein, ZNF592. Eur. J. Hum. Genet. EJHG 18, 1107–1113 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vodopiutz J. et al. WDR73 Mutations Cause Infantile Neurodegeneration and Variable Glomerular Kidney Disease. Hum. Mutat. 36, 1021–1028 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parikshak N. N. et al. Integrative functional genomic analyses implicate specific molecular pathways and circuits in autism. Cell 155, 1008–1021 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi M., Weidenheim K. M., Dickson D. W. & Ksiezak-Reding H. Morphological and biochemical correlations of abnormal tau filaments in progressive supranuclear palsy. J. Neuropathol. Exp. Neurol. 61, 33–45 (2002). [DOI] [PubMed] [Google Scholar]
- 62.Gg K. et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol. (Berl.) 140, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roemer S. F. et al. Rainwater Charitable Foundation criteria for the neuropathologic diagnosis of progressive supranuclear palsy. Acta Neuropathol. (Berl.) 144, 603–614 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beecham G. W. et al. The Alzheimer’s Disease Sequencing Project: study design and sample selection. Neurol. Genet. 3, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuzma A. et al. NIAGADS: The NIA Genetics of Alzheimer’s Disease Data Storage Site. Alzheimers Dement. 12, 1200–1203 (2016). [Google Scholar]
- 66.Lowy-Gallego E. et al. Variant calling on the GRCh38 assembly with the data from phase three of the 1000 Genomes Project. Wellcome Open Res. 4, 50 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.for the Alzheimer’s Disease Neuroimaging Initiative et al. Linkage, whole genome sequence, and biological data implicate variants in RAB10 in Alzheimer’s disease resilience. Genome Med. 9, 100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Genome Reference Consortium. GRCh38 reference 000001405.15. https://ftp.ncbi.nlm.nih.gov/genomes/all/GCA/000/001/405/GCA_000001405.15_GRCh38/seqs_for_alignment_pipelines.ucsc_ids/GCA_000001405.15_GRCh38_no_alt_analysis_set.fna.gz.
- 69.Schneider V. A. et al. Evaluation of GRCh38 and de novo haploid genome assemblies demonstrates the enduring quality of the reference assembly. Genome Res. 27, 849–864 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manichaikul A. et al. Robust relationship inference in genome-wide association studies. Bioinformatics 26, 2867–2873 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conomos M. P., Miller M. B. & Thornton T. A. Robust inference of population structure for ancestry prediction and correction of stratification in the presence of relatedness. Genet. Epidemiol. 39, 276–293 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gogarten S. M. et al. Genetic association testing using the GENESIS R/Bioconductor package. Bioinformatics 35, 5346–5348 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mc Cartney A. M. et al. Chasing perfection: validation and polishing strategies for telomere-to-telomere genome assemblies. Nat. Methods 19, 687–695 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zou Y., Carbonetto P., Wang G. & Stephens M. Fine-mapping from summary data with the “Sum of Single Effects” model. PLoS Genet. 18, e1010299 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang K., Li M. & Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McLaren W. et al. The Ensembl Variant Effect Predictor. Genome Biol. 17, 122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cunningham F. et al. Ensembl 2022. Nucleic Acids Res. 50, D988–D995 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Karczewski K. J. et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581, 434–443 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee S. et al. Optimal Unified Approach for Rare-Variant Association Testing with Application to Small-Sample Case-Control Whole-Exome Sequencing Studies. Am. J. Hum. Genet. 91, 224–237 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee Seunggeun, Zhao Zhangchen, Miropolsky Larisa, & Wu Michael. SKAT: SNP-Set (Sequence) Kernel Association Test. (2020). [Google Scholar]
- 81.Sjöstedt E. et al. An atlas of the protein-coding genes in the human, pig, and mouse brain. Science 367, eaay5947 (2020). [DOI] [PubMed] [Google Scholar]
- 82.Melé M. et al. Human genomics. The human transcriptome across tissues and individuals. Science 348, 660–665 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Langfelder P. & Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9, 559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen X. et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–1222 (2016). [DOI] [PubMed] [Google Scholar]
- 85.Layer R. M., Chiang C., Quinlan A. R. & Hall I. M. LUMPY: a probabilistic framework for structural variant discovery. Genome Biol. 15, 1–19 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eggertsson H. P. et al. GraphTyper2 enables population-scale genotyping of structural variation using pangenome graphs. Nat. Commun. 10, 1–8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang H. et al. Structural Variation Detection and Association Analysis of Whole-Genome-Sequence Data from 16,905 Alzheimer’s Diseases Sequencing Project Subjects. medRxiv (2023). [Google Scholar]
- 88.Scherer S. W. et al. Challenges and standards in integrating surveys of structural variation. Nat. Genet. 39, S7–S15 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amemiya H. M., Kundaje A. & Boyle A. P. The ENCODE blacklist: identification of problematic regions of the genome. Sci. Rep. 9, 1–5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Belyeu J. R. et al. Samplot: a platform for structural variant visual validation and automated filtering. Genome Biol. 22, 1–13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thorvaldsdóttir H., Robinson J. T. & Mesirov J. P. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief. Bioinform. 14, 178–192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Purcell S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
NIAGADS Data Sharing Service (https://dss.niagads.org/)
https://github.com/whtop/PSP-Whole-Genome-Sequencing-Analysis