Figure 7.
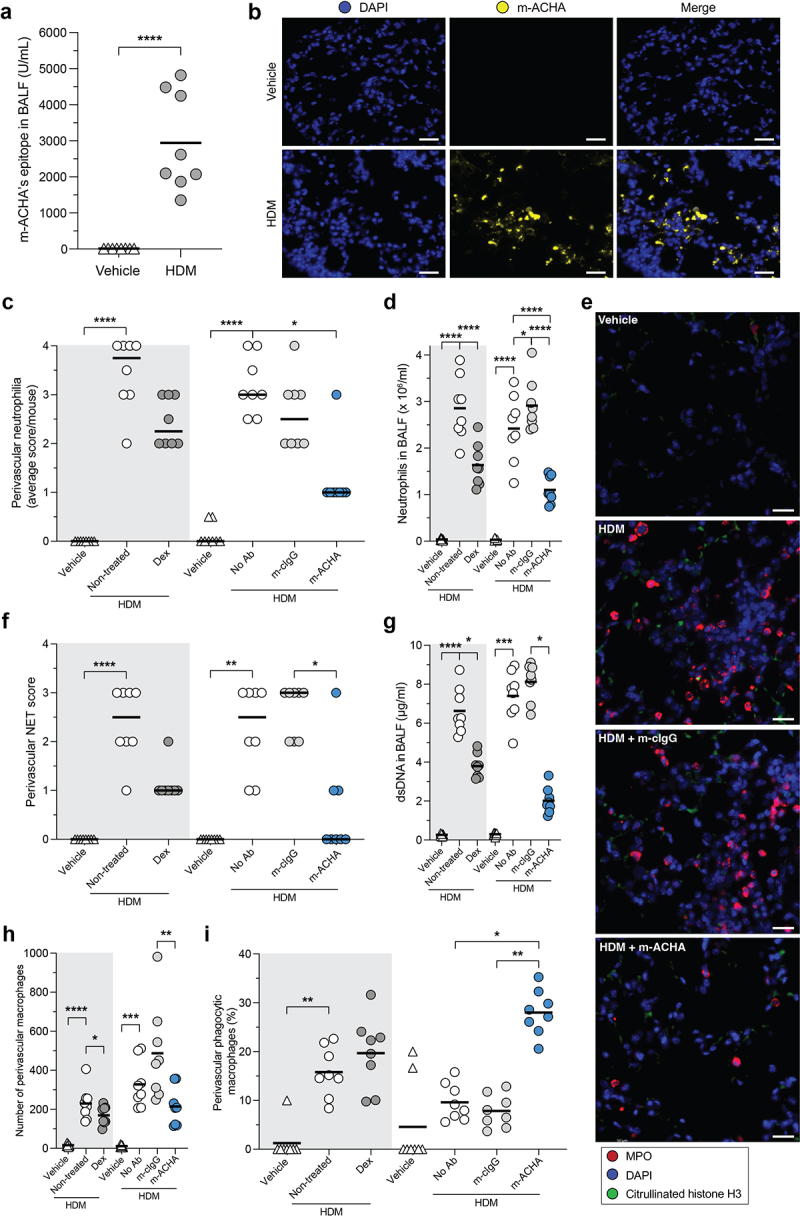
Reduced inflammatory infiltrate and enhanced phagocytosis induced by m-ACHA in a neutrophilic airway inflammation mouse model.
CFA/HDM-sensitized Balb/c mice were challenged (i.n.) with HDM or vehicle. One hour before challenge, mice received Dex or vehicle p.o. (gray area) or received m-ACHA, mouse isotype control antibody (m-cIgG), or vehicle i.v. (white area). BALF and lung tissue were collected 24 hours after challenge. (a) m-ACHA epitope levels in BALF from vehicle and HDM challenged mice which did not receive any antibody treatment. (b) Representative fluorescence IHC images from lung tissue of vehicle and HDM challenged mice stained with DAPI (DNA in blue) and m-ACHA (yellow). The scale bar is 20 µm. (c) Semi-quantitative scores of perivascular neutrophilia in H&E-stained lung sections (2 sections per mouse). (d) Neutrophil numbers in BALF. (e) Anatomically matched and randomly selected fluorescence IHC images from lung tissue stained with anti-citrullinated histone H3 antibody (green), anti-MPO antibody (red), and DAPI. The scale bar is 20 µm. (f) Semi-quantitative scoring of NETs (admixing of citH3 and MPO) in the perivascular zone of immunofluorescent-stained lung sections. Autofluorescence signals were present in images (green channel), but NETs were considered extracellular based on the morphology or minimal distance of 3 nuclear radii from the nearest nucleus. (g) dsDNA levels in BALF. The total number of macrophages (h) and the percentage of phagocytic macrophages (i) in the perivascular zone. Graphs are depicted as median in (c and f) and as mean in (a, d, and g−i) of eight mice per group. Comparisons were made per administration route (p.o. or i.v.). *P <.05, **P <.01, ***P <.001, ****P <.0001, unpaired two-tailed t test (a), Kruskal-Wallis test with Dunn’s multiple comparison test (c and f−h), and ordinary one-way ANOVA with Holm-Šidák’s multiple comparisons test (d and i).
