FIG 2.
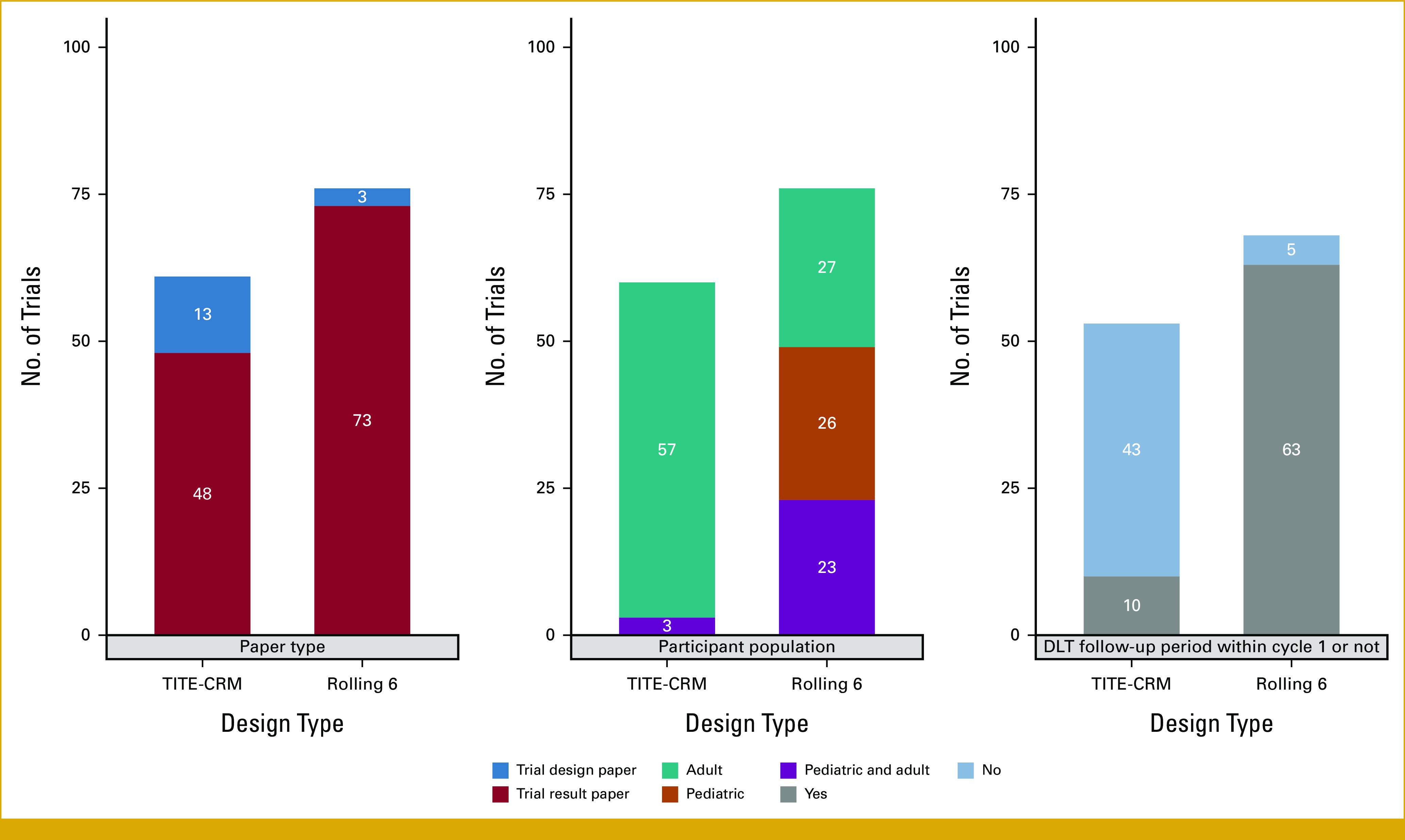
Basic characteristics of trials using TITE-CRM and rolling 6. DLT, dose-limiting toxicity; TITE-CRM, time-to-event continuous reassessment method.

Basic characteristics of trials using TITE-CRM and rolling 6. DLT, dose-limiting toxicity; TITE-CRM, time-to-event continuous reassessment method.