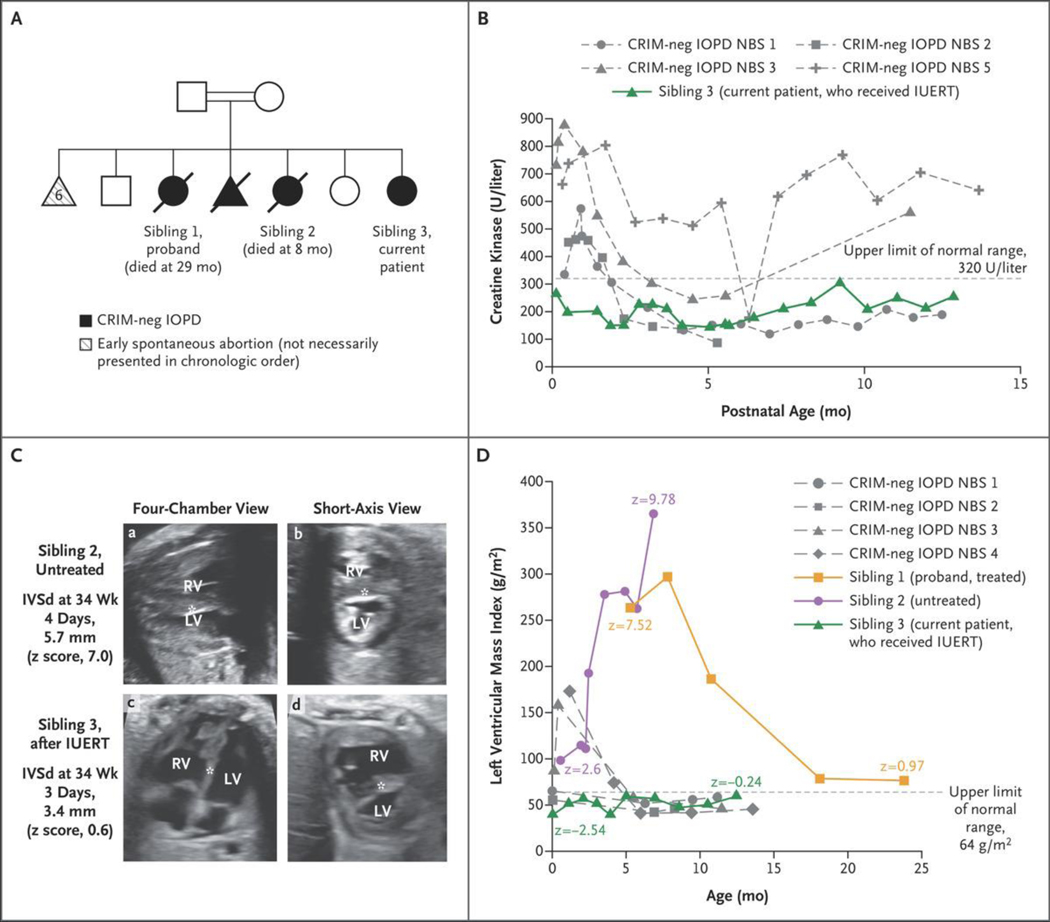
Figure 1. Case Presentation and Skeletal-Muscle and Cardiac Outcomes.
Panel A shows the family pedigree. Squares indicate male family members, circles female family members, triangles pregnancies not carried to term, double bars consanguinity, open symbols unaffected, filled black symbols affected, and diagonal slashes deceased. CRIM denotes cross-reactive immunologic material, IOPD infantile-onset Pompe’s disease, and neg negative. Panel B shows creatine kinase levels at 4 days or more of life in patients with CRIM-negative IOPD (gray dashed curves) treated after newborn screening (NBS) (treated at ≤4 weeks of age)7 as compared with the levels in Sibling 3 (green). IUERT denotes in utero enzyme-replacement therapy. Panel C shows fetal echocardiograms, four-chamber (a and c) and short-axis (b and d), of Sibling 2, who was untreated (top row), and Sibling 3, who received IUERT (bottom row) performed at 34 weeks of gestation. The ventricular-wall thickness, quantified as diastolic measurement of the interventricular septum (IVSd) (asterisk), was 5.7 mm (z score, 7.0) in Sibling 2, whereas it remained normal in S ibling 3 (3.4 mm; z score, 0.6) during fetal therapy. The z scores were calculated according to the methods of Firpo et al.18 LV denotes left ventricle, and RV right ventricle. Panel D shows the left ventricular mass index in Sibling 3 (green) as compared with patients with CRIM-negative IOPD treated after NBS (gray dashed curves)7 and the patient’s two previous affected siblings (Siblings 1 [orange] and 2 [purple]). The first data point for Sibling 1 is just before the initiation of enzyme-replacement therapy (ERT), and the plot shows prolonged time to improvement; Sibling 2 never received ERT.