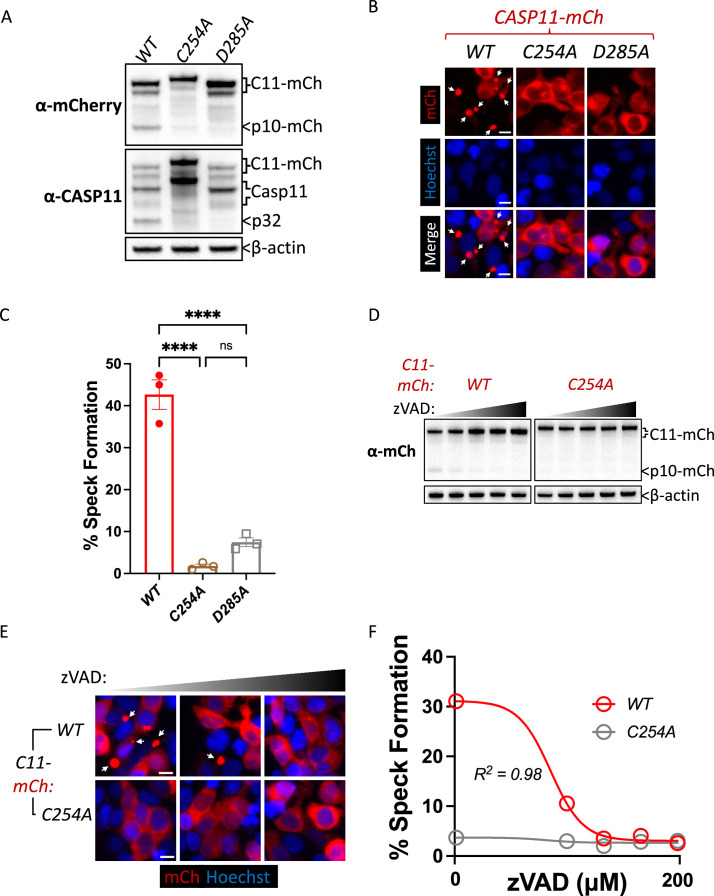
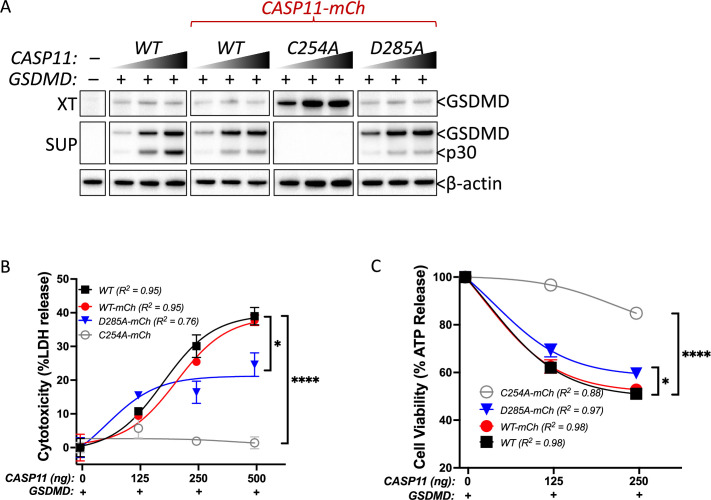
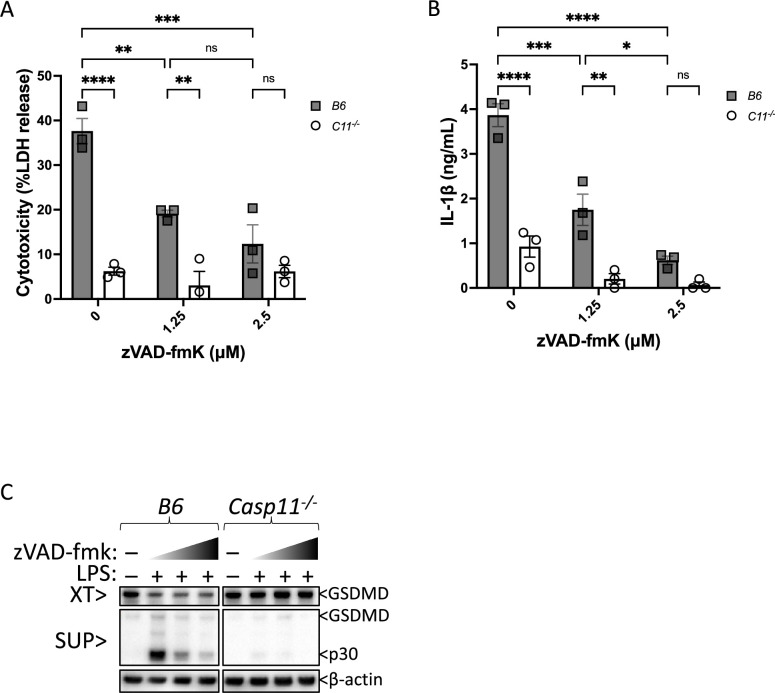
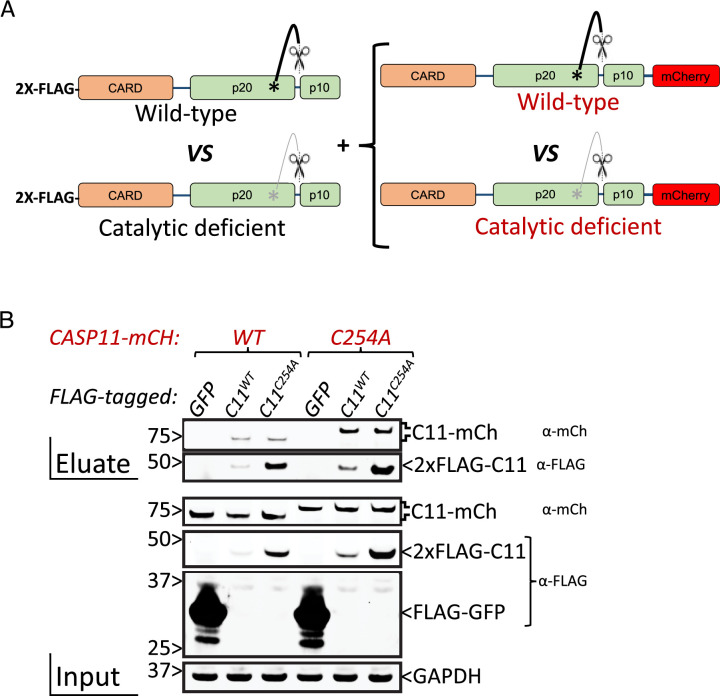
Figure 2. Caspase-11 catalytic activity and autoprocessing at the interdomain linker are required for spontaneous caspase-11 oligomerization in HEK293T cells.
(A) Wild-type (WT), catalytically inactive (C254A), and non-cleavable (D285A) Casp11-mCherry expression plasmids were transfected (0.25 μg) into HEK293T cells. Cell lysates were immunoblotted for mCherry, Casp11, and β-actin (loading control) 10 hr post-transfection. (B) HEK293T cells were transfected with wild-type (WT), catalytically inactive (C254A), or non-cleavable (D285A) Casp11-mCherry as described in ‘Materials and methods’ and imaged by fluorescence microscopy 18 hr post-transfection. Nuclei (blue) were stained with Hoechst, white arrows denote Casp11-mCherry specks. Scale bar = 10 μm. (C) Speck formation in (B) was quantified as percentage of Casp11-mCherry-expressing cells containing at least one speck. (D) HEK293T cells were transfected with Casp11-mCherry constructs as in (B) and 6 hr post-transfection, and cells were incubated with increasing amounts of pan-caspase inhibitor zVAD (0–200 μM; twofold increments). Whole-cell lysates were isolated 12 hr post-transfection and immunoblotted for mCherry or β-actin loading control as indicated. Cleaved p10-mCherry is denoted. (E) Casp11-mCherry speck formation was assayed in zVAD-treated cells by fluorescence microscopy as in (B). (F) Speck formation in (E) was quantified as percentage of Casp11-mCherry-expressing cells containing at least one speck. Dose–response curves in (F) were plotted by least-squares nonlinear regression ([Log2(inhibitor) vs. response (three parameters)]; Y = Bottom + (Top-Bottom)/(1 + 10(X-LogIC50)); R2 indicated). Error bars represent mean ± SEM of triplicate wells (800–900 cells per well); representative of 2–3 independent experiments. Bar graphs in (C) were analyzed by two-way ANOVA with Sidak’s multiple-comparison test, ***p<0.001.