Table 5.
AR Affinities and Selectivities of Tricyclic Purinone Derivatives
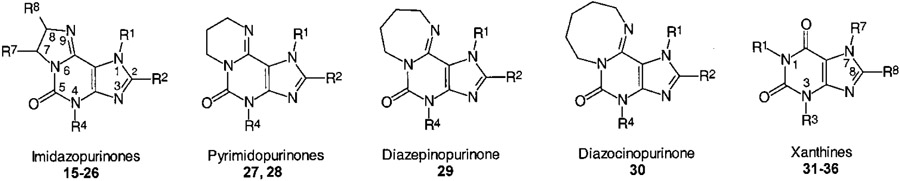
|
||||||||
|---|---|---|---|---|---|---|---|---|
| compd | R1 | R2 | R4 | R7 | R8 |
Ki (μM) ± SEM or % displacement at indicated concentration (in brackets) |
||
| A1 affinity rat brain cortical membranes1 [3H]CHA |
A2A affinity rat brain striatal membranes1 [3H]CGS21680 |
A3 affinity human recombinant receptors [125I]AB-MECA |
||||||
| 2-Unsubstituted Imidazopurinones | ||||||||
| 15 | methyl | H | H | H | H | 65.5 ± 7.6 | 40.9 ± 6.3 | >10a |
| 16 | methyl | H | benzyl | H | H | 24% (30 μM) | 16.1 ± 3.8 | ndb |
| 17 | methyl | H | methyl | H | H | 79.6 ± 0.6 | 20.7 ± 3.0 | 40% (10 μM) |
| R-18 | methyl | H | methyl | H | (R)-methyl | 14% (3 μM) | 12% (30 μM) | nd |
| S-18 | methyl | H | methyl | H | (S)-methyl | 10% (10 μM) | 46% (30 μM) | nd |
| RS-19 | methyl | H | methyl | H | (R,S)-ethyl | 46% (250 μM) | 16.2 ± 0.6 | 28% (100 μM) |
| R-19 | methyl | H | methyl | H | (R)-ethyl | 75.5 ± 4.5 | 28.3 ± 5.4 | nd |
| S-19 | methyl | H | methyl | H | (S)-ethyl | 50% (250 μM) | 10.9 ± 1.2 | nd |
| RS-20 | methyl | H | methyl | (R/S)-methyl | H | 59 ± 8 | 64.6 ± 9.8 | >10a |
| R-20 | methyl | H | methyl | (R)-methyl | H | 23 ± 4 | 50.4 ± 13.4 | nd |
| S-20 | methyl | H | methyl | (S)-methyl | H | 70 ± 6 | 12.0 ± 1.9 | nd |
| 2-Substituted Imidazopurinones | ||||||||
| 21 | methyl | phenyl | H | H | H | 1.83 ± 0.9 | 11.9 ± 1.6 | 0.047 ± 0.0048 |
| 22 | methyl | phenyl | methyl | H | H | 9.7 ± 2.6 | 20.3 ± 2.6 | 3.33 ± 0.72 |
| RS-23 | methyl | phenyl | methyl | H | (R,S)-ethyl | 6.1 + 0.8 | 19.2 ± 3.9 | 17% (10 μM) |
| R-23 | methyl | phenyl | methyl | H | (R)-ethyl | 17.5 ± 5.0 | 36.3 ± 1.2 | nd |
| S-23 | methyl | phenyl | methyl | H | (S)-ethyl | 4.7 ± 0.3 | 16.2 ± 4.6 | nd |
| RS-24 | H | phenyl | methyl | H | (R,S)-ethyl | 0.265 ± 0.1 | 3.1 ± 0.5 | nd |
| R-24 (PSB-11) | H | phenyl | methyl | H | (R)-ethyl | 0.44 ± 0.1 | 2.1 ± 0.14 | 0.0023 ± 0.0011 |
| S-24 | H | phenyl | methyl | H | (S)-ethyl | 0.115 + 0.001 | 3.33 ± 0.93 | 0.0098 ± 0.0038 |
| R-25 | methyl | styryl | methyl | H | (R)-ethyl | 21.7 ± 3.8 | 0.547 ± 0.035 | 1.70 ± 0.48 |
| S-25 | methyl | styryl | methyl | H | (S)-ethyl | 14.9 ± 2.6 | 0.424 ± 0.007 | 30.6 ± 8.3 |
| R-26 | H | styryl | methyl | H | (R)-ethyl | 0.95 ± 0.15 | 0.89 ± 0.01 | 0.64 ± 0.199 |
| S-26 | H | styryl | methyl | H | (S)-ethyl | 0.73 ± 0.13 | 0.67 ± 1.73 | >10a |
| Ring-Enlarged Analogues of Imidazopurinones | ||||||||
| 27 | methyl | H | methyl | 43.2 ± 13.4 | 28.5 ± 5.2 | >10a | ||
| 28 | methyl | Cl | methyl | 10.5 ± 6.1 | 11.9 ± 0.87 | nd | ||
| 29 | methyl | H | methyl | 19.8 ± 9.5 | 18.5 ± 1.3 | >10a | ||
| 30 | methyl | H | methyl | 60.3 ± 8.1 | 51 ± 4 | >10a | ||
| Xanthine Derivatives (for Comparison) | ||||||||
| R7 | R8 | R3 | R1 | name | ||||
| 31 | methyl | H | methyl | methyl | caffeine | 4139 | 4339 | 13.37 |
| 32 | methyl | phenyl | methyl | methyl | 8-phenylcaffeine | 1540 | 2540 | nac |
| 33 | H | phenyl | methyl | methyl | 8-phenyltheophylline | 0.08939 | 0.8339 | na |
| 34 | methyl | styryl | methyl | methyl | 8-styrylcaffeine | 3.941 | 0.09441 | na |
| 35 | H | styryl | methyl | methyl | 8-styryltheophylline | 0.6541 | 0.2941 | na |
nd = not determined.
Less than 10% displacement at 10 μM.
Data not available.
