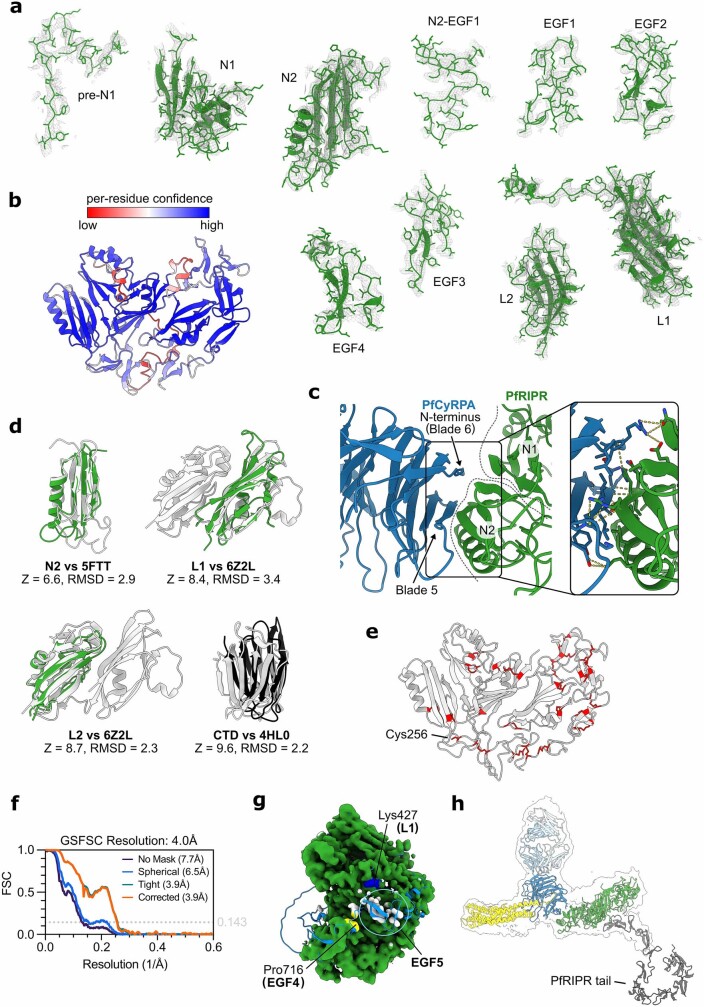
Extended Data Fig. 7. The PfRIPR core and generating a composite model of full-length PfRIPR.
a, Domains of PfRIPR with their corresponding cryo-EM density using the PfRCR-Cy.003 composite map post-processed with DeepEMhancer. b, AlphaFold2 prediction of PfRIPR residues 19–716 (except 484–548) coloured by pLDDT score. The regions of low confidence in this model correlate with those that required de novo building guided by the AlphaFold2 prediction. c, The interface between PfRIPR and PfCyRPA comprises backbone and side chain interactions between residues of domains N1 and N2 of PfRIPR (green) and blades 5 and 6 of PfCyRPA (blue). Each are shown as cartoons with side chains of interacting residues (detailed in Extended Data Table 4) shown as sticks in the expanded section. Hydrogen bonds between residues are shown in yellow. d, Structural alignment of PfRIPR domains and their structural homologues with their DALI Z scores and RMSD scores. Structural homologues are identified by their PDB codes: 5FTT (lectin domain of Lactrophilin 3), 6Z2L (PfP113) and 4HL0 (Galectin). e, The PfRIPR core contains 23 disulphide bonds and one free cysteine (Cys256). Cysteine residues are shown as red sticks. f, Gold standard Fourier shell correlation (GSFSC) curves for the PfRCR-Cy.003 map containing additional density for the start of the PfRIPR tail. g, The PfRIPR portion of the composite PfRCR-Cy.003 map coloured green where a model of the PfRIPR core has been built. Uninterpretable density (coloured white) is observed in this map beyond domain EGF4 at Pro716 (yellow). An AlphaFold2 model of PfRIPR truncated after EGF5 (residues 20–769, light blue cartoon) predicts that this likely corresponds to EGF5. This is further supported by a chemical crosslink found between Lys736 of domain EGF5 and Lys427 of domain L1 (dark blue) in XL-MS analysis of the PfRCR complex (Extended Data Fig. 3). h, To generate a composite model of full-length PfRIPR, the AlphaFold 2 prediction of the PfRIPR tail (residues 717–1086) was docked onto the PfRIPR core using the 4.0 Å PfRCR-Cy.003 map containing additional PfRIPR density as a guide.