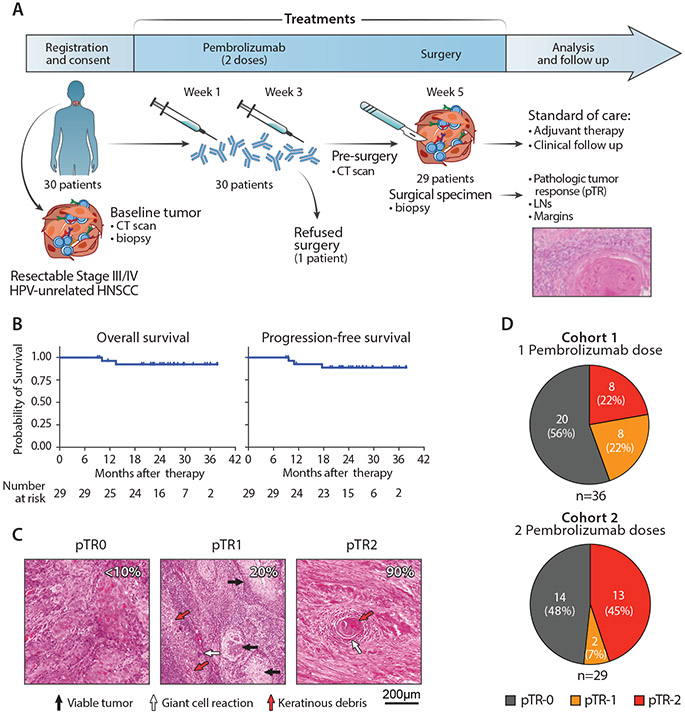
Fig. 1. Outcome of neoadjuvant PD-1 blockade in patients with HNSCC.
(A) Scheme of treatment and outcome analysis. After enrollment and screening, patients with HNSCC received 2 doses of anti-PD-1 before surgery. Resected tumors specimens were assessed for the rate of response as part of pathologic staging. (B) Kaplan-Meier curves estimating the overall survival (left) and progression-free survival (left) of 29 patients with HNSCC who received 2 doses of neoadjuvant PD-1 blockade and surgery. Time from surgical resection is presented as months elapsed. The number of evaluated at-risk patients is reported below each graph. (C) Hematoxylin-eosin staining of tumor biopsies collected at surgery from 3 representative patients with different levels of pTR (quantification indicated by white numbers). Arrows - representative areas with viable tumor cells (black), giant cell/histiocytic reaction (white) or necrotic cells/keratinous debris (red). (D) Rates of pTR in patients treated with 2 neoadjuvant doses of anti-PD-1 antibodies (this study, right, n=29) compared with patients who received a single dose, as previously reported (3) (left).