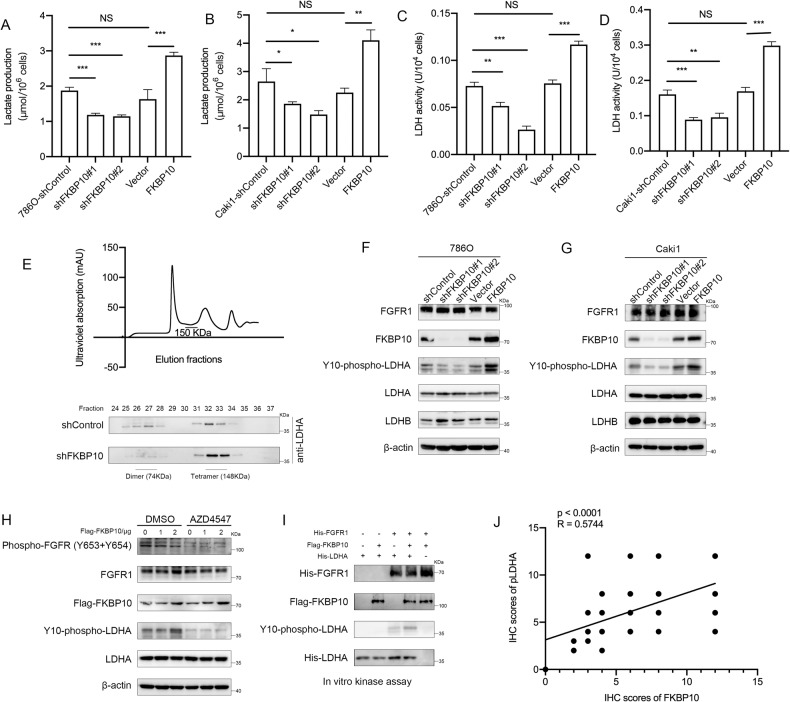
Fig. 4. FKBP10 promotes the phosphorylation of the LDHA-Y10 site.
A–D Lactate production levels and LDH enzyme activity in FKBP10-overexpressing or FKBP10-knockdown cells were examined as indicated. Data are presented as the means ± SD, and were independently replicated three times. E Gel filtration analysis of whole lysates from 3 × 106 786 O cells stably transfected with control shRNA or shFKBP10 shRNA. Western blot analysis of LDHA protein levels in various fractions collected via gel filtration. F, G FKBP10 regulated the phosphorylation of LDHA-Y10 in 786 O and Caki1 cells. H FKBP10 regulated the phosphorylation of LDHA in a FGFR1-dependent manner. I Purified recombinant LDHA and FKBP10 proteins were incubated with or without active recombinant FGFR1 in the in vitro kinase assay. J The IHC scores of LDHA-Y10 phosphorylation were positively correlated with FKBP10. Statistical analyses were performed with a two-tailed unpaired Student’s t test (NS, P > 0.05, * P < 0.05, **P < 0.01, ***P < 0.001).