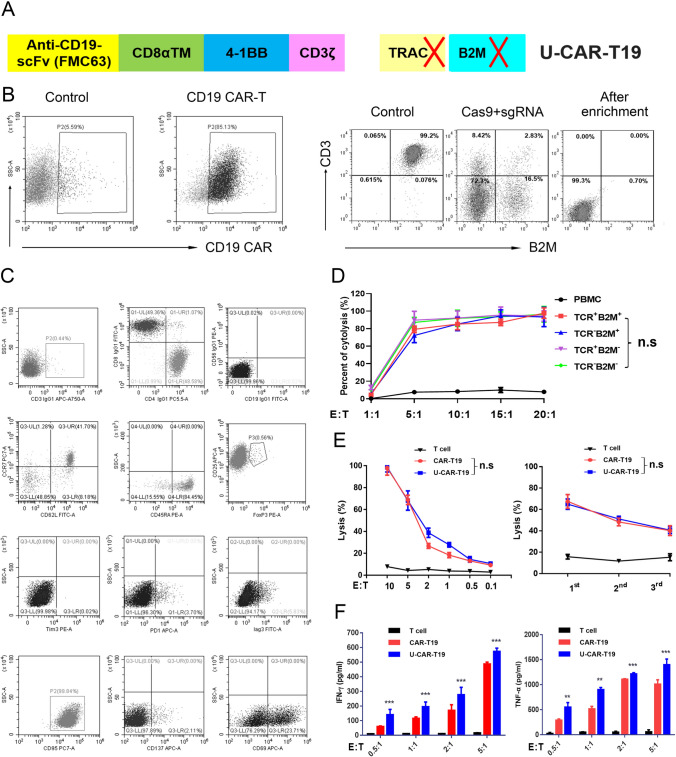
Fig. 1.
Construction of TRAC−/B2M− allogeneic CAR-T19 cells using the CRISPR/Cas9 method. a The second-generation anti-CD19 CARs were constructed with antibody clone FMC63, hinge and transmembrane regions from CD8, 4-1BB (CD137), and CD3ζ. Then, TRAC and B2M were knockout by CRISPR/Cas9 to get U-CAR-T19 cells. b Representative images of CAR-T19 and U-CAR-T19 cells. CAR-T19 cells were electroporated with RNP comprising of Cas9 and sgRNAs that target TRAC and B2M loci and cultured for 7 days. Both membrane CD3 complex and B2M expression were detected by flow cytometry following enrichment and purification with magnetic beads to remove CD3+ CAR-T cells. c Expression of markers associated with T cell activation, exhaustion, memorization, and regulatory (CD56, CCR7, CD62L, CD45RA, CD25, FoxP3, Tim-3, PD-1, LAG3, CD95, CD137, and CD69) were detected by flow cytometry. d TCR and/or B2M-deficient cells were stimulated with Raji cells at the given effector/target (E/T) ratios. The 24 h cytolysis of effector cells to target cells was detected by measuring the release of LDH. e Specific lysis of U-CAR-T19 cells at different E/T ratios and different rounds of stimulation with target cells (1:1 for 24 h). CAR-T19 and U-CAR-T19 cells were co-cultured with Raji cells to perform the cytolysis assay, and the E/T ratios and stimulation are shown in the figure. The cytolysis of effector cells to target cells was detected by measuring the release of LDH. (F) IFN-γ and TNF-α expressions were determined by ELISA after co-culture of CAR-T19/U-CAR-T19 and target cells (18 h). Statistical significance, ANOVA with Tukey’s multiple comparison test or two-tailed, paired Student’s t test were used, n.s, no significance, *P < 0.05, **P < 0.01, ***P < 0.001