Abstract
Background:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with increased morbidity and mortality in solid organ transplant (SOT) recipients. Despite exclusion from SARS-CoV-2 vaccine clinical trials, these individuals were identified as high-risk and prioritized for vaccination in public health guidelines.
Methods:
We prospectively evaluated humoral and cellular immune responses to two doses of the SARS-CoV-2 mRNA vaccine, BNT162b2, in 56 SOT recipients and 26 healthy controls (HCs). Blood specimens collected from participants prior to each dose and following the second dose were tested for SARS-CoV-2-specific antibodies, as well as CD4+ and CD8+ T-cell responses.
Results:
SOT recipients demonstrated lower mean anti-SARS-CoV-2 antibody levels compared to HCs after each dose, and only 21.6% achieved an antibody response after the second dose within the range of HC responses. Similarly, the percentage of responsive CD4+ and CD8+ T cells in SOT recipients was lower than in HCs. While most HCs showed notable humoral and cellular responses, responses were less concordant in SOT recipients, with some showing evidence of either humoral or cellular response, but not both.
Conclusion:
Humoral and cellular immune responses to the BNT162b2 vaccine are markedly reduced in SOT recipients as compared to HCs, suggesting that SOT recipients may benefit from more tailored regimens such as higher dose and/or additional vaccinations.
Keywords: immune responses, SARS-CoV-2 vaccine, solid organ transplant
1 |. INTRODUCTION
Despite pervasive availability in the United States of highly effective vaccines for severe acute respiratory syndrome coronavirus 2 (SARSCoV-2), the etiology of coronavirus disease 2019 (COVID-19), special populations continue to be significantly impacted by infection. These prominently include solid organ transplant (SOT) recipients, who experience increased incidence of severe COVID-19 and higher mortality rates due to SARS-CoV-2 infections.1–4
Given the urgency to develop and implement effective measures to prevent SARS-CoV-2-associated morbidity and mortality in the general population, multiple vaccines were rapidly developed shortly after the pandemic was declared.5 Favorable results from phase III clinical trials evaluating vaccine efficacy and safety in healthy individuals led to the wide rollout of three vaccines to the general US population under Emergency Use Authorization by the US Food and Drug Administration (FDA).6–8 These viral spike protein-expressing vaccines included two mRNA vaccines, BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna, Inc.), and one adenoviral vector vaccine, Ad26.CoV2.S (Janssen/J&J).9–11 However, SOT recipients were excluded from SARS-CoV-2 vaccine clinical trials.9,10,12
Recently, the FDA fully approved BNT162b2 and authorized a third dose of mRNA vaccines for SOT recipients based on reports demonstrating an immune response among a subset of patients unresponsive to the standard two-dose series.13,14 Longitudinal evaluation of immune responses among SOT recipients to currently approved SARS-CoV-2 vaccines is therefore imperative, especially given suboptimal responses to standard and augmented vaccination regimens.15–19
Here, we report the results of a prospective cohort study comparing immunogenicity and reactogenicity associated with two doses of the BNT162b2 (Pfizer-BioNTech) vaccine between SOT recipients and healthy controls (HCs).
2 |. METHODS
2.1 |. Study population and design
Participants were enrolled under a generic vaccine immunogenicity and safety protocol. This study was part of a larger prospective cohort study of individuals receiving any SARS-CoV-2 vaccine. Eligible individuals included SOT recipients and HCs who were scheduled to receive two doses of the BNT162b2 vaccine as part of routine medical care at Vanderbilt University Medical Center (Nashville, TN). For this analysis, due to targeted vaccination of elderly individuals from the general population and healthcare workers who are comparatively younger and represent most of our HCs, persons were excluded if they were under 55 years of age. Additional exclusion criteria were acute illness (e.g., fever, respiratory symptoms, and/or gastrointestinal symptoms) within 48 h of initial study visit, laboratory-confirmed SARS-CoV-2 infection prior to receiving the first vaccine dose, or previous vaccination against SARS-CoV-2. HCs were also excluded if they had an underlying medical condition (UMC) requiring immunosuppressive therapy. Participants who acquired laboratory-confirmed SARS-CoV-2 infection were excluded. The institutional review board of Vanderbilt University approved the study, and participants were enrolled after providing written informed consent.
Demographic, social, and clinical data were collected, and medical chart abstractions were performed to verify and obtain clinical and transplant history data, where applicable. UMCs were collected and classified in the following categories: autoimmune disease, cardiovascular disease, chronic kidney disease, chronic liver disease, chronic lung disease, diabetes mellitus, dyslipidemia, hypertension, malignancy, and obesity. All data were entered into a standardized, secured REDCap (Research Electronic Data Capture, Vanderbilt University, Nashville, TN, USA) database.20,21
Blood specimens were obtained at the following time points: visit 1 (0–2 days before first vaccine dose), visit 2 (21–42 days after first vaccine dose), and visit 3 (21–42 days after second vaccine dose). HCs had two additional visits, supplemental visits 1 and 2 (5–10 days after first and second vaccine doses, respectively). Nasal swabs were collected during visits 1 and 2. Figure S1 illustrates study visits and procedures.
2.2 |. Laboratory testing of blood specimens
Immunoglobulin G (IgG) to SARS-CoV-2 spike receptor-binding domain (RBD), spike extracellular domain (ECD), and nucleocapsid protein (N) was evaluated by enzyme-linked immunosorbent assay (ELISA). Anti-N IgG served as a serologic marker of prior or intercurrent SARS-CoV-2 infection since the vaccine lacks any portion of N. Quantification of binding IgG against the RBD of SARS-CoV-2 was further performed on participant sera using a liquid bead-array assay as previously described.22 Detailed ELISA procedures are available in the Appendix.
Cellular immune responses were quantified using an antigeninduced marker assay. Cryopreserved peripheral blood mononuclear cells were thawed in the presence of nuclease S7 (Sigma Aldrich [Roche], St. Louis, MO) and rested overnight at 37°C in Roswell Park Memorial Institute medium with 10% human type AB serum (R10). The following day, cells were counted, washed with R10, and resuspended at 10 ×106 cells/ml. Anti-CD40 blocking antibody (Miltenyi Biotech, Auburn, CA) was added to cells at a final concentration of 0.5 μg/ml (1:200 dilution) for 15 min. Cells were then added to wells of a 96-well plate (one million cells per stimulation condition), and anti-CD154 (CD49L) Phycoerythrin (PE) antibody was added to each well at a 1:10 final dilution. Cells were stimulated with the following antigens: Genscript SARS CoV-2 ECD (Cat#Z03481) at a final concentration of 10 μg/ml, Genscript SARS CoV-2 NP (i.e., N) (Cat#Z03488) at a final concentration of 10 μg/ml, a pool of overlapping peptides spanning the SARS CoV-2 ECD at a final concentration of 1 μg/ml per peptide (Cat#NR-52402, BEI Resources, Manassas, VA), or staphylococcal enterotoxin B (Millipore Sigma, Burlington, MA) at a final dilution of 1:100. After overnight stimulation, cells were stained with the following antibody panel: anti-CD8 APC-Cy, -CD137 APC, -CD3 AF700, -CD45RO PE CF594, -OX40 PE-Cy7, -CD4 PcPCy5.5, -CD69 BV605, and -CD25 BV786 and LD aqua V500. CD4+ responses to each antigen were defined as %CD4+OX40+CD154+ or %CD8+CD25+CD137+.
2.3 |. Testing for SARS-CoV-2
Nasal swabs were collected to document asymptomatic SARS-CoV-2 detection via reverse-transcription quantitative polymerase chain reaction (RT-qPCR). Anterior nares were sampled using polyester flocked swabs (Puritan Medical Products), which were immediately placed in PrimeStore Molecular Transport Medium (Longhorn Vaccines and Diagnostics) and maintained at room temperature until RT-qPCR testing for SARS-CoV-2 using methods described in the CDC EUA protocol, CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel.23,24 Detailed procedures are available in the Appendix.
2.4 |. Reactogenicity and safety assessments
Research personnel acquired data on reactions to vaccination via patient interview. For SOT recipients, specific local symptoms (pain, tenderness, swelling, or redness at the injection site) and systemic symptoms (fever, fatigue, headache, nausea, vomiting, or myalgia) and highest symptom grade (mild, moderate, or severe) were documented during visits 2 and 3. For HCs, specific local and systemic symptoms were documented at the supplemental visits, while any local or systemic symptoms at any time and corresponding highest symptom grade were documented on visits 2 and 3. Mild symptoms were defined as being noticeable but not limiting daily activities. Moderate symptoms were those that limited daily activities. Severe symptoms were those that prevented daily activities. Chart reviews were conducted for SOT recipients to evaluate acute cellular and antibody rejection episodes occurring within 14 days of each dose.
2.5 |. Statistical analysis
Descriptive statistics were summarized as absolute and relative frequency for categorical variables and mean (standard deviation) or median (interquartile range) for continuous variables, as appropriate. All analyses were conducted using R version 4.0.3. Robust (heteroscedasticity-consistent) standard errors were used in all regression models, and significance was determined to be achieved at the (two-sided) α = .05 level. Eligible participants with detectable baseline of antibodies against SARS-CoV-2 N (defined as an assay value exceeding six standard deviations of the group mean, highly suggesting prior infection)25 were excluded from our analyses of immunogenicity and reactogenicity data; however, these individuals were descriptively evaluated independently from the analyses described in this section.
Humoral responses were analyzed as follows. Each ELISA was corrected for background (blank wells) and standardized to the platespecific (and background-corrected) pooled negative control (PNC). To compare humoral immune responses to the BNT162b2 vaccine (both between SOT recipients and HCs at each time point and within each group over time), we used generalized estimating equations with a working independence correlation structure, with (background-corrected, PNC-standardized) ELISA anti-RBD, -ECD, and -N IgG levels as three separate outcomes each measured in ELISA units (EU). Each model was adjusted for prevaccination (baseline) seroreactivity. Anti-RBD IgG levels based on Luminex were measured in world health organization (WHO) international units per milliliter (IU/ml), and an analogous model was fit with the outcome log-transformed and coefficients exponentiated to yield geometric mean ratios. For each regression model, we performed a sensitivity analysis in which age and gender were included as adjustment covariates.
Participants having ELISA anti-ECD IgG levels at least as high as the minimum visit 3 value among HCs were classified as antibody responders (“seropositive”; by this definition, all HCs were deemed antibody responders after the second dose). We sought to evaluate factors associated with mounting a humoral immune response among SOT recipients; we used Pearson’s χ2 test to compare the proportion of vaccine responders between groups defined by age (>70 vs. ≤70 years), gender, organ transplant type (liver vs. other), immunosuppressive regimen (comparing calcineurin inhibitor [CNI] alone to CNI + other drugs, and comparing mycophenolic acid [MPA] to no MPA), and time post-transplant (>1 vs. ≤1 year).
Cellular immune responses were analyzed as follows. For CD4 and CD8 assays, the percentage of activated cells in the media-control condition was subtracted from the percentage under each antigenstimulation condition. To compare cellular immune responses between SOT recipients and HCs, we used linear regression to estimate the differences in mean (media-corrected) percentage of CD4+ T cells responsive to ECD (%CD4+OX40+CD154+), percentage of CD8+ T cells responsive to ECD (%CD8+CD25+CD137+), and percentage of CD8+ T cells responsive to ECD peptides (%CD8+CD25+CD137+), each adjusted for baseline percentage of activated cells. We fit an age and gender-adjusted model as a sensitivity analysis analogous to the one described for humoral responses.We used generalized estimating equations with a working independence correlation structure to estimate themean rise in cellular responseswithinHCs and SOTrecipients.
To evaluate reactogenicity, we used Pearson’s χ2 test to compare the proportion of SOT recipients and HCs reporting any local or systemic symptoms following each vaccination. We further descriptively evaluated and compared symptom severity between SOT recipients and HCs.
3 |. RESULTS
3.1 |. Study population
From December 18, 2020 to March 7, 2021, 258 participants were enrolled in the larger prospective cohort study. Among them, 80 (54 SOT and 26 HCs) participants met inclusion criteria for this study (Figure S2). Three participants (all SOT recipients) were excluded from the immunogenicity (and reactogenicity) analyses due to detectable baseline levels of antibodies against SARS-CoV-2 N protein. The median time between first and second vaccine doses was 21 days (range: 21–28) among SOT recipients and 21 days (range: 21–24) among HCs.
SOT recipients had a higher mean age and were more likely to be male as compared to HCs (Table 1). Almost all SOT recipients had at least one UMC, with hypertension and dyslipidemia being most common. The most common organ transplant types were kidney (22/54; 41%) and liver (20/54, 37%). All SOT participants were on CNIs. CNI alone and a combination of CNI, MPA, and corticosteroids were the most frequent maintenance immunosuppressive regimens (Table 1). MPA was included in the immunosuppressive regimens of 24 of 54 (44.4%) SOT recipients. The median time posttransplant at the first vaccination was 7.2 (interquartile range: 2.7–13.0) years (Table 1).
TABLE 1.
Baseline demographic and clinical characteristics of the SOT and HC groups
| Characteristics | SOT recipients (N = 54) | HC (N = 26) |
|---|---|---|
| Mean age (SD) — years | 72.1 (3.6) | 62.4 (6.7) |
| Male sex — number (%) | 33 (61.1) | 9 (34.6) |
| Race and ethnicity— number (%) | ||
| White non-Hispanic | 48 (88.9) | 23 (88.5) |
| Other non-Hispanic | 6 (11.1) | 3 (11.5) |
| Mean body mass index (SD) — kg/m2 | 28.1 (5.4) | 27.7 (5.6) |
| Underlying medical conditions — number (%) | ||
| Any underlying medical condition | 53 (98.2) | 18 (69.2) |
| Hypertension | 47 (87.0) | 8 (30.8) |
| Dyslipidemia | 39 (72.2) | 9 (34.6) |
| Chronic kidney disease | 48 (88.9) | 0 (0.0) |
| Cardiovascular disease | 30 (55.6) | 4 (15.4) |
| Diabetes mellitus | 27 (50.0) | 2 (7.7) |
| Obesity | 17 (31.5) | 7 (26.9) |
| Chronic lung disease | 11 (20.4) | 3 (11.5) |
| Autoimmune disease | 7 (13.0) | 2 (7.7) |
| Malignancy | 6 (11.1) | 0 (0.0) |
| Chronic liver disease | 4 (7.4) | 0 (0.0) |
| Non-immunosuppressive medications — number (%) | 54 (100) | 19 (73.1) |
| Organ type — number (%) | ||
| Kidney | 22 (40.7) | – |
| Liver | 20 (37.0) | – |
| Heart | 6 (11.1) | – |
| Lung | 4 (7.4) | – |
| Kidney and liver | 2 (3.7) | – |
| Immunosuppressive medications — number (%) | ||
| CNI only | 12 (22.2) | – |
| CNI, corticosteroids, and MPA | 12 (22.2) | – |
| CNI and corticosteroids | 11 (20.4) | – |
| CNI and MPA | 9 (16.7) | – |
| CNI, corticosteroids, and azathioprine | 3 (5.6) | – |
| CNI and mTOR inhibitors | 2 (3.7) | – |
| CNI and azathioprine | 4 (7.4) | – |
| CNI, corticosteroids, and mTOR inhibitors | 1 (1.9) | – |
| Median time since transplant (IQR) — years | 7.0 (2.7–13.0) | – |
Abbreviations: CNI, calcineurin inhibitor; HC, healthy control; IQR, interquartile range; mTOR, mammalian target of rapamycin; MPA, mycophenolic acid; SD, standard deviation; SOT, solid organ transplant.
3.2 |. SARS-CoV-2 detection
We collected and tested 23 of 26 and 24 of 26 nasal swab specimens from HCs at visits 1 and 2, respectively. From SOT recipients, we collected and tested 51 of 54 nasal swabs at visit 1 and 50 of 54 nasal swabs at visit 2. SARS-CoV-2 was not detected in any specimen. Invalid results were obtained for a single specimen obtained from an SOT recipient, which lacked detectable RNase P (an endogenous cellular positive control) and SARS-CoV-2 target amplification in two independent total nucleic acid extracts.
3.3 |. Humoral responses
SOT recipients manifested markedly lower titers of anti-RBD and anti-ECD IgG by ELISA and anti-RBD IgG by Luminex when compared to HCs (Figure 1 and Figure S3, respectively). Adjusted mean differences in anti-RBD and anti-ECD IgG levels between SOT recipients and HCs are summarized in Table 2; all between-group mean differences were found to be statistically significant. Although both HCs and SOT recipients demonstrated a significant rise in mean anti-spike IgG following the second dose as compared to the first, the magnitude of rise was substantially higher in HCs, with HCs having an estimated 0.59 EU higher mean rise in anti-RBD IgG levels (95% CI: [0.34, 0.83]; p <.001) and a 0.39 EU higher mean rise in anti-ECD IgG levels (95% CI: [0.19, 0.59]; p <.001); the group-specific mean changes are presented in Table S1. Sensitivity analysis including adjustments for age and gender did not result in conclusions that differed from our main analyses (data not shown).
FIGURE 1.
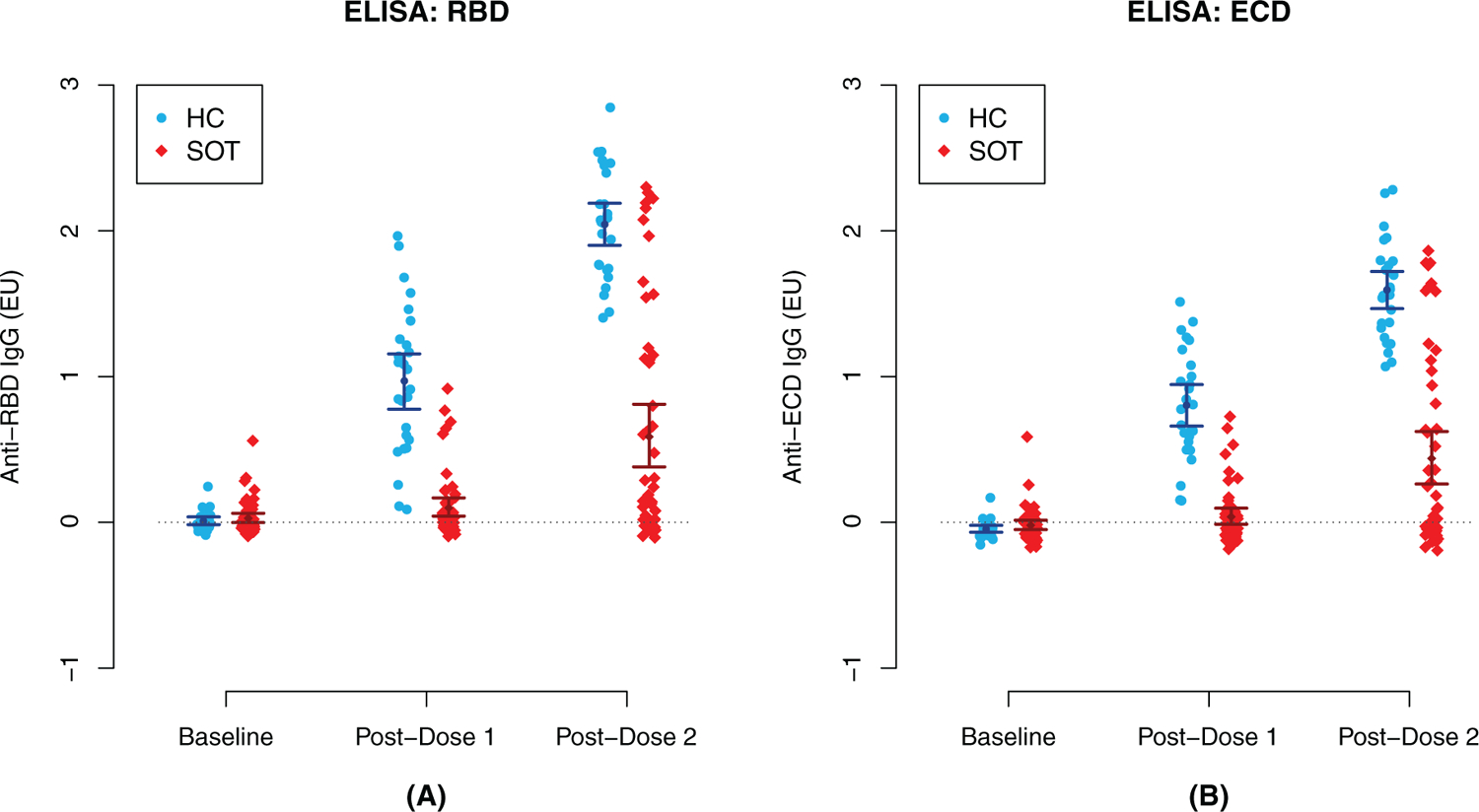
Enzyme-linked immunosorbent assay (ELISA) anti-receptor binding domain (RBD) (A) and anti-extracellular domain (ECD) (B) severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG antibody levels at baseline, postdose 1 and postdose 2 in healthy controls (HCs) and solid organ transplant (SOT) recipients. Included are unadjusted point estimates and 95%Confidence intervals (CIs). All adjustedmean differences in antibody responses between SOT recipients and HCs were statistically significant postdose 1 and postdose 2 (p < .001)
TABLE 2.
Baseline-adjusted comparisons of humoral and cellular responses between SOT recipients and HCs following each vaccination (reported as adjusted mean differences between SOT recipients and HCs unless otherwise noted)
| Postvaccine 1 | Postvaccine 2 | |
|---|---|---|
| ELISA IgG to RBD (EU) | −0.89 (−1.09, −0.68); p < .001 | −1.48 (−1.74,−1.22); p < .001 |
| ELISA IgG to ECD (EU) | −0.78 (−0.94, −0.63); p < .001 | −1.17 (−1.39, −0.95); p < .001 |
| Luminex IgG to RBD (IU/ml)† | 0.015 (0.008,0.028); p < .001 | 0.007 (0.003 0.018); p < .001 |
| CD4+ T-cell ECD response (%) | n/a | −0.28 (−0.51, −0.042); p = .021 |
| CD8+ T-cell ECD response (%) | n/a | −0.14 (−0.28,0.001); p = .052 |
| CD8+ T-cell peptide response (%) | n/a | −0.069 (−0.15,0.016); p = .11 |
Abbreviations: ECD, extracellular domain; EU, enzyme-linked immunosorbent assay (ELISA) units; HCs, healthy controls; IgG, immunoglobulin G; IU, international units; RBD, receptor binding domain; SOT, solid organ transplant.
Adjusted geometric mean ratio between SOT recipients and HCs (unitless; 1<indicates lower response among SOT recipients).
The threshold for defining antibody response (i.e., the minimum value of anti-ECD IgG among HCs following the second dose) was estimated to be 1.07 EU. Following the first vaccine dose, seven of 26 (26.9%) of HCs were classified as antibody responders by this metric, as compared to zero of 51 (0.0%) of SOT recipients. Following the second dose, 26 of 26 (100%) HCs were responders (by construction) compared to 11 of 51 (21.6%) of SOT recipients. Of the 11 SOT subjects classified as antibody responders, eight were liver transplant recipients, and three were kidney transplant recipients.
We found that SOT recipients receiving treatment with MPA had a lower estimated proportion of vaccine responders as compared to those treated with other immunosuppressive drugs (0/21 [0.0%] as compared to 11/30 [36.7%]; p = .002). Further, SOT recipients receiving CNI treatment alone had a higher proportion of vaccine responders as compared to those receiving CNI in conjunction with other immunosuppressive drugs (5/11 [45.5%] as compared to 6/40 [15.0%]; p = .030). Non-liver transplant recipients had a lower proportion of vaccine responders as compared to liver recipients (3/33 [9.1%] as compared to 8/18 [44.4%]; p = .003). Furthermore, only one of 18 (6%) liver transplant recipients received MPA compared to 20 of 33 (61%) non-liver transplant recipients. There was insufficient evidence of an association between age, sex, or time post-transplant and vaccine response.
3.4 |. Cellular responses and relationship with humoral responses
CD4 and CD8 responses to ECD were evaluated in n = 51 subjects (n = 27 SOT recipients and n = 24 HCs); CD8 responses to peptides were evaluated in n = 44 subjects (n = 22 SOT recipients and n=22 HCs). Cellular responses are presented for each group at baseline and following the second dose in Figure S4. The mean rise in %CD4+T cells responsive to ECD from baseline was statistically significant among HCs (0.30%; 95% CI: [0.14, 0.45]; p < .001), though not among SOT recipients (–0.027%; 95% CI: [–0.19, 0.14]; p = .75). The estimated in mean difference %CD4+ T cells responsive to ECD was 0.28% lower among SOT recipients (95% CI: [0.042, 0.51]; p = .021) following the second dose.
The mean rise in %CD8+ T cells responsive to peptides from baseline was statistically significant among HCs (0.10%; 95% CI: [0.038, 0.17]; p = .002), though not among SOT recipients (0.047%; 95% CI: [–0.014, 0.11]; p = .13). The estimated mean difference in %CD8+ T cells responsive to peptides was 0.069% lower among SOT recipients (95% CI: [–0.016, 0.15]; p=.11) Between-group differences in CD8+ T cell responses to ECD are reported in Table 2 and follow similar trends. In our sensitivity analysis including age and sex as adjustment variables, analogous trends held but were not statistically significant.
We noted strong concordance between changes in cellular and humoral immune responses from baseline to visit 3 in the HC group (Figure 2); mean humoral and cellular responses showed significant rises from baseline to visit 3 (p < .001 for each). While mean humoral responses showed a significant rise from baseline to visit 3 in the SOT group (p < .001), cellular responses did not (p = .75 for CD4+ T cells and p = .13 for CD8+ T cells). Of the three SOT individuals with evidence of cellular responses (>0.5% at visit 3), two were liver transplant recipients, and one was a kidney transplant recipient. Only the kidney transplant recipient had a concurrent antibody response.
FIGURE 2.
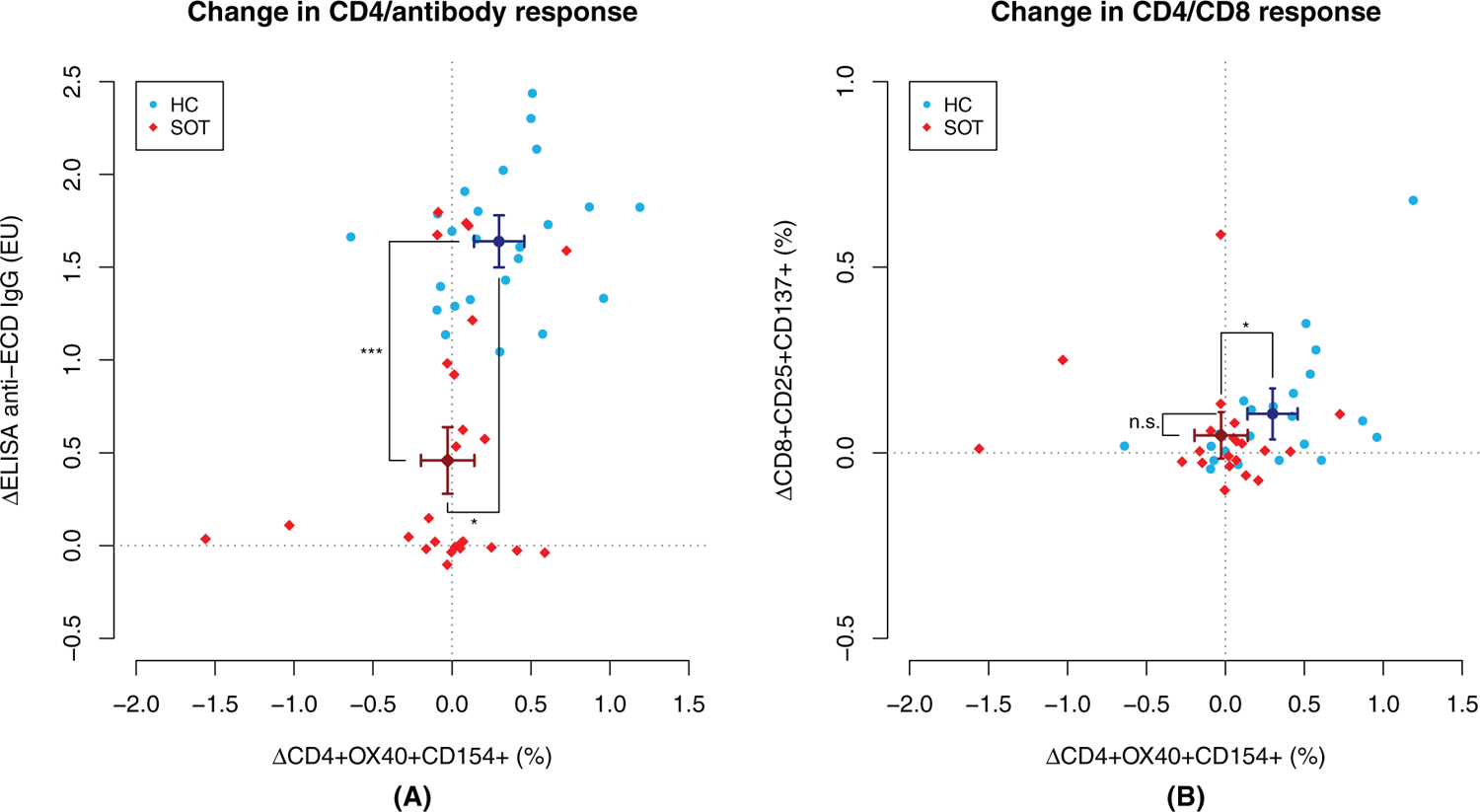
Changes in cellular and humoral immune responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) extracellular domain (ECD) between baseline and visit 3 in healthy controls (HCs) and solid organ transplant (SOT) recipients. In panel (A), we present the change in %CD4+ T cells responsive to extracellular domain (ECD) on the x-axis and change in IgG response to ECD on the y-axis. In panel (B), the y-axis represents change in %CD8+ T cells responsive to peptides. Also depicted in each plot are the point estimates and respective 95% CIs for each assay
3.5 |. Reactogenicity and safety assessments
Following the first dose, similar percentages of SOT recipients and HCs reported any local or systemic symptoms (33/51 [64.7%] vs. 18/26 [69.2%], p = .69). After the second dose, a lower percentage of SOT recipients reported any local or systemic symptoms when compared to HCs (31/51 [60.8%] vs. 22/26 [84.6%], p=.033). Symptom grades were similar in both groups after each dose. The most frequently reported local symptoms in HCs were pain at the injection site after the first dose and tenderness at the injection site after the second dose. The most frequent systemic symptom in HCs was fatigue after both the first and second doses (Figure S5A). Tenderness at the injection site and fatigue were the most frequently reported local and systemic symptoms, respectively, in SOT recipients after each dose (Figure S5B).
No acute cellular or antibody rejection events were noted among SOT recipients within 14 days of either vaccine dose.
3.6 |. Subjects excluded due to baseline seropositivity
Humoral immune responses to vaccination among the three SOT recipients excluded from the primary analysis for reasons of baseline SARS-CoV-2 seropositivity are depicted in Figure S6. The participant with the lowest baseline anti-RBD antibody level did not respond to the first dose and showed only a minimal rise in anti-RBD antibody after the second dose. This individual was a 71-year-old male liver transplant recipient 5.7 years posttransplant and maintained on a CNI. The participant reported no local or systemic symptoms after either dose. The subject with the highest baseline anti-RBD antibody level was a 74-year-old female liver transplant recipient 18 years posttransplant and maintained on CNI and azathioprine. A modest and plateauing increase in anti-RBD IgG was observed after the first dose, with negligible further rise after the second dose. This person reported mild symptoms after the first dose only. The third participant was a 72-year-old male kidney transplant recipient 7.5 years posttransplant and maintained on steroids and CNI. A moderate and plateauing increase in anti-RBD level, with no additional rise after the second dose, was observed. Only mild symptoms after each dose were reported by this subject.
4 |. DISCUSSION
In our prospective vaccine study comparing two doses of BNT162b2 SARS-CoV-2 mRNA vaccine between SOT recipients and HCs, we found markedly attenuated humoral and cellular responses among SOT recipients after administration of both vaccine doses. Relative to HCs, SOT recipients developed lower mean anti-RBD and anti-ECD antibody levels. While a second dose boosted antibody titers in both groups, the magnitude of increase was lower in SOT recipients. Unlike HCs, we also noted weaker CD4+ T-cell responses in SOT recipients associated with a major discordance between cellular and humoral immune responses, where antibody responses were observed in the absence of detectable cellular responses and vice versa. SOT recipients who received an organ other than liver and those receiving a combination of immunosuppressive medications were less likely to respond, most straightforwardly explained by lower intensity of immunosuppression associated with liver transplantation.
Our findings of dampened humoral immune responses to BNT162b2 vaccine among SOT recipients are consistent with other recent reports on seroresponse rates ranging from 22%–58.8% after two doses of SARS-CoV-2 mRNA vaccines in this population.18,19,26–29 We observed a seropositivity rate of 21.6% with the SOT cohort. Similarly, Korth et al. reported positive antibody responses to two doses of the BNT162b2 vaccine in 22% of 23 renal transplant recipients, whose mean age was 57.7 years compared to 72.1 years in our SOT cohort.26 None of the SOT recipients in our study population responded to a single dose, and although mean anti-RBD and anti-ECD IgG titers among SOT recipients increased following a second dose, the response was greatly diminished relative to HCs. Boyarsky et al. similarly reported an antibody response in 39% of 658 SOT recipients after the second dose despite no response to the first dose of either the BNT162b2 or mRNA-1273 vaccine.30,31 Recent studies have demonstrated significant improvement in humoral responses following a third dose of SARS-CoV-2 mRNA vaccines among some SOT recipients; however, a large proportion remain seronegative.32–34 Although immune correlates of protection following SARS-CoV-2 mRNA vaccination of SOT recipients are undetermined, available data indicate an important protective role by antibodies to spike protein.35–38 Our results underscore the continuing need to evaluate the capacity of additional doses or hybrid prime-boost regimens to improve antibody production in the setting of anti-rejection immunosuppression and to understand mechanistically how alternative strategies achieve their effects.39
In our HC cohort, postdose 2 (visit 3) CD4+ T-cell responses to SARS-CoV-2 ECD were readily detectable and paralleled RBD and ECD antibody production as well as CD8+ T-cell responses to ECD peptides. Prior studies have simultaneously evaluated cellular and humoral immune responses to mRNA vaccines and demonstrated CD4+ and CD8+ T-cell responses by intracellular cytokine staining in HCs, including individuals >70 years of age.11,40,41 Compared to antibody responses, evaluation of cellular immune responses to SARS-CoV-2 mRNA vaccination has been much more limited in SOT recipients.18,19,42,43
We evaluated expression of lymphocyte activation markers after antigen stimulation similar to our prior work44 and that of other groups evaluating cellular responses to SARS-CoV-2 after natural infection or vaccination.18,45 In contrast to HCs, SOT recipients demonstrated extremely blunted cellular responses to ECD, which further lacked correlation with individual antibody levels. Miele et al. found diminished cellular immune responses to BNT162b2 vaccine in SOT patients as measured by IFN-γ ELISPOT assay.42 Sattler et al. evaluated humoral and cellular immune responses to BNT162b2 vaccine in 39 kidney transplant recipients and 39 immunocompetent controls and reported significantly lower levels of vaccine-induced antigen-specific serum IgG and IgA along with reduced CD4+ and CD8+ T-cell responses in the SOT group compared to controls. Consistent with our results, other studies have demonstrated discordant cellular and humoral immune responses among organ transplant recipients. Cucchiari et al. evaluated mRNA-1273 vaccine immunogenicity among 162 kidney transplant recipients, in which 14 participants generated an antibody response in the absence of a detectable cellular response, while 41 participants exhibited the opposite pattern.43 More recently, Hall et al. described discordant cellular and humoral immune responses after mRNA-1273 vaccination.19 Thus, our findings and those from other groups support a working model of weakened or functionally aberrant cell-mediated responses to vaccination in SOT recipients, resulting in diminished antibody production. Importantly, absence of a measurable humoral response to the SARS-CoV-2 vaccine does not necessarily imply lack of protection from infection, as cell-mediated responses may also play a role.38 There are several potential mechanisms for impaired cellular immune responses after SOT, including effects of common anti-rejection regimens on molecular programming of T-helper primary and recall responses, efficiency of T-cell interactions with B lymphocytes and other antigen-presenting cells, maintenance of the T-cell receptor repertoire, and alterations of activation thresholds in naïve and memory T-helper populations. Further investigation is needed to define critical T-cell functions that may explain poor humoral responses in this population.
This study has some limitations. First, the SOT group was significantly older than HCs. While older age could have influenced vaccine responses independently of immune status, BNT162b2 and mRNA-1273 vaccines induce high titers of neutralizing antibodies in older individuals.41,46 To address the potential confounding effect of age, we adjusted for this covariate when comparing humoral responses in a sensitivity analysis and found results consistent with our main analyses. The notable difference in age distribution between SOT recipients and HCs largely explains discrepant results of analyses of cellular immune responses before and after adjusting for age. We also note that the majority of subjects in our sample were white. At the time of data collection, vaccine eligibility for non-immunocompromised individuals was based on age, and therefore the overall demographic breakdown of our sample is more representative of that of older individuals rather than that of the general population Second, our available sample size was unable to accommodate an in-depth mechanistic exploration of individual factors associated with diminished immune responses within the SOT group; however, we were able to demonstrate the association between immunosuppressive drug regimen and antibody response. Third, we did not measure neutralizing antibodies specifically; however, levels of mRNA-1273-induced binding antibody to spike and RBD correlate with protection from COVID-19 in immunocompetent hosts.38 Fourth, we acknowledge that seropositivity was defined subjectively and is not intended to serve as a correlate of protection against SARS-CoV-2 infection. Seropositivity provides a metric for comparing vaccine immunogenicity between SOT recipients and HCs, and these measurements may serve as a reference for gauging vaccine responses in other studies examining immunogenicity and efficacy of mRNA vaccines in SOT recipients. Fifth, we acknowledge that seropositivity was defined subjectively and not intended to serve as a correlate of protection against SARS-CoV-2 infection. Seropositivity provides a metric for comparing vaccine immunogenicity between SOT recipients and HCs, and these measurements might serve as a reference for gauging vaccine responses in other studies examining immunogenicity and efficacy of mRNA vaccines in SOT recipients. Sixth, direct comparison of reactogenicity assessments between SOT and HC participants may be subject to potential recall and measurement biases given that severity data were collected at different time points and frequencies for the two groups. Finally, we note that the majority of subjects in our SOT cohort were white, which is not representative of the Vanderbilt SOT recipient population. In 2021, almost 67% of the transplant recipients at VUMC were white, whereas 88% of the enrolled SOT recipients were white, this limits our generalizability. Since this was early on in the vaccine rollout, it is consistent with the racial/ethnic disparities reported in vaccine uptake.47 Special strengths of this study include its prospective design, which allowed longitudinal evaluation of vaccine responses following each dose; patient and visit-level paired serologic and cellular analyses for an integrated picture of the adaptive immune response to vaccination; and companion HC group as a benchmark for natural evolution of humoral and cellular responses to SARS-CoV-2 mRNA vaccine.
Results from our study document poor humoral and cellular immune responses to SARS-CoV-2 mRNA vaccines in SOT recipients, which likely places them at increased risk for infection and disease. Therefore, these individuals should continue practicing non-pharmaceutical interventions, such as social distancing and masking, as an adjunct to vaccination. There remains a pressing research and medical need to identify strategies capable of overcoming impaired immune responses to SARS-CoV-2 vaccines in SOT recipients to provide durable protection against disease in this high-risk population.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. Ryan Stark (VUMC) for helping with development of the SARS-CoV-2 ELISA used for this study. Additionally, we thank Heather O’Dell (VUMC) who helped with recruitment coordination of SOT recipients. This work was supported by the Craig Weaver Endowment Chair awarded to NBH. DAR is supported by the National Institutes of Health (award number: TL1TR002244). SAK, CHN, CMW, JDS, and Eric Olsen ECO were supported by R01AI142095. The TN-CFAR Laboratory Science Core (P30AI110527) helped isolate cells and plasma from study participants. Natasha Halasa (NBH) receives grant support from Sanofi and Quidel and speaker compensation from an education grant supported by Genentech. Sanofi also donated vaccines and influenza antibody testing for an influenza vaccine trial.
Funding information
The TN-CFAR Laboratory Science Core, Grant/Award Number: P30AI110527; National Institutes of Health, Grant/Award Numbers: TL1TR002244, R01AI142095; Craig Weaver Endowment Chait awarded to Natasha Halasa
Abbreviations:
- CNI
calcineurin inhibitors
- COVID-19
coronavirus disease 2019
- ECD
extracellular domain
- ELISA
enzyme-linked immunosorbent assay
- EU
ELISA unit
- FDA
food and drug administration
- HCs
healthy controls
- IgG
immunoglobulin G
- MPA
mycophenolic acid
- N
nucleocapsid protein
- RBD
receptor binding domain
- RT-qPCR
reverse-transcription quantitative polymerase chain reaction
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SOT
solid organ transplant
- TNA
total nucleic acid
- UMC
underlying medical condition
Footnotes
CONFLICT OF INTEREST
All the authors have no conflict of interest to disclose.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Azzi Y, Parides M, Alani O, et al. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020;98(6):1559–1567. 10.1016/j.kint.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher AM, Schlauch D, Mulloy M, et al. Outcomes of COVID-19 in hospitalized solid organ transplant recipients compared to a matched cohort of non-transplant patients at a national healthcare system in the United States. Clin Transplant. 2021;35:e14216. 10.1111/ctr.14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair V, Jandovitz N, Hirsch JS, et al. An early experience on the effect of solid organ transplant status on hospitalized COVID-19 patients. Am J Transplant. 2020;21(7):2522–2531. 10.1111/ajt.16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yi SG, Rogers AW, Saharia A, et al. Early experience with COVID-19 and solid organ transplantation at a US high-volume transplant center. Transplantation. 2020;104(11):2208–2214. 10.1097/tp.0000000000003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awadasseid A, Wu Y, Tanaka Y, Zhang W. Current advances in the development of SARS-CoV-2 vaccines. Int J Biol Sci. 2021;17(1):8–19. 10.7150/ijbs.52569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration Moderna COVID-19 vaccine EUA letter of authorization. 2021. Accessed May 5, 2021.
- 7.U.S. Food and Drug Administration Janssen COVID-19 Vaccine EUA letter of authorization. 2021. Accessed May 5, 2021.
- 8.U.S. Food and Drug Administration Pfizer-BioNTech COVID-19 vaccine EUA letter of authorization. 2021. Accessed May 5, 2021.
- 9.Janssen Biotech Inc Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. 2021. https://www.fda.gov/media/146217/download. Accessed May 5, 2021.
- 10.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383(20):1920–1931. 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Administration USFD Approval Letter. 2021. https://www.fda.gov/media/151710/download. Accessed August 25, 2021
- 14.FDA Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals. 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised. Accessed August 25, 2021
- 15.Hirzel C, Kumar D. Influenza vaccine strategies for solid organ transplant recipients. Curr Opin Infect Dis. 2018;31(4):309–315. 10.1097/qco.0000000000000461. [DOI] [PubMed] [Google Scholar]
- 16.Eckerle I, Rosenberger KD, Zwahlen M, Junghanss T. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8(2):e56974. 10.1371/journal.pone.0056974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangappa S, Wrammert J, Wang D, et al. Kinetics of antibody response to influenza vaccination in renal transplant recipients. Transpl Immunol. 2019;53:51–60. 10.1016/j.trim.2019.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sattler A, Schrezenmeier E, Weber UA, et al. Impaired humoral and cellular immunity after SARS-CoV2 BNT162b2 (Tozinameran) prime-boost vaccination in kidney transplant recipients. J Clin Invest. 2021;131(14):e150175. 10.1172/jci150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall VG, Ferreira VH, Ierullo M, et al. Humoral and cellular immune response and safety of two-dose SARS-CoV-2 mRNA-1273 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21:3980–3989. 10.1111/ajt.16766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett M, Yoder S, Brady E, et al. A high-throughput liquid bead array assay confirms strong correlation between SARS-CoV-2 antibody level and COVID-19 severity. iScience. 2021;24(2):102052. 10.1016/j.isci.2021.102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prevention CfDCa CDC 2019-novel coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel. 2020. https://www.fda.gov/media/134922/download. Accessed May 11, 2021 [DOI] [PMC free article] [PubMed]
- 24.Rha B, Lively JY, Englund JA, et al. Severe acute respiratory syndrome coronavirus 2 infections in children: multicenter surveillance, United States, January–March 2020. J Pediatr Infect Dis Soc. 2020;9(5):609–612. 10.1093/jpids/piaa075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dörschug A, Frickmann H, Schwanbeck J, et al. Comparative assessment of sera from individuals after S-gene RNA-based SARS-CoV-2 vaccination with spike-protein-based and nucleocapsid-based sero-logical assays. Diagnostics. 2021;11(3):426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korth J, Jahn M, Dorsch O, et al. Impaired humoral response in renal transplant recipients to SARS-CoV-2 vaccination with BNT162b2 (Pfizer-BioNTech). Viruses. 2021;13(5):756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grupper A, Rabinowich L, Schwartz D, et al. Reduced humoral response to mRNA SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients without prior exposure to the virus. Am J Transplant. 2021;21(8):2719–2726. 10.1111/ajt.16615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benotmane I, Gautier-Vargas G, Cognard N, et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021;99(6):1498–1500. 10.1016/j.kint.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marinaki S, Adamopoulos S, Degiannis D, et al. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am J Transplant. 2021;21(8):2913–2915. 10.1111/ajt.16607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–1786. 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyarsky BJ, Werbel WA, Avery RK, et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. 10.1001/jama.2021.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamar N, Abravanel F, Marion O, Couat C, Izopet J, Del Bello A. Three doses of an mRNA covid-19 vaccine in solid-organ transplant recipients. N Engl J Med. 2021;385(7):661–662. 10.1056/NEJMc2108861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Bello A, Abravanel F, Marion O, et al. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am J Transplant. 10.1111/ajt.16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021;326(11):1063–1065. 10.1001/jama.2021.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630–634. 10.1038/s41586-020-03041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–2040. 10.1101/2021.06.21.21258528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sui Y, Bekele Y, Berzofsky JA. Potential SARS-CoV-2 immune correlates of protection in infection and vaccine immunization. Pathogens. 2021;10(2):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert PB, Montefiori DC, McDermott A, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. medRxiv. 2021. 10.1101/2021.08.09.21261290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Werbel WA, Boyarsky BJ, Ou MT, et al. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174(9):1330–1332. 10.7326/l21-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 41.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miele M, Busà R, Russelli G, et al. Impaired anti-SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am J Transplant. 2021;21(8):2919–2921. 10.1111/ajt.16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cucchiari D, Egri N, Bodro M, et al. Cellular and humoral response after mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients. Am J Transplant. 2021;21(8):2727–2739. 10.1111/ajt.16701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pilkinton MA, McDonnell WJ, Barnett L, et al. In chronic infection, HIV gag-specific CD4+ T cell receptor diversity is higher than CD8 T+ cell receptor diversity and is associated with less HIV quasispecies diversity. J Virol. 2021;95(8):e02380–20. 10.1128/JVI.02380-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):eabf4063. 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Services USDoHH Organ procurement and transplantation network. https://optn.transplant.hrsa.gov/data/view-data-reports/state-data/#. Accessed November 8, 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


