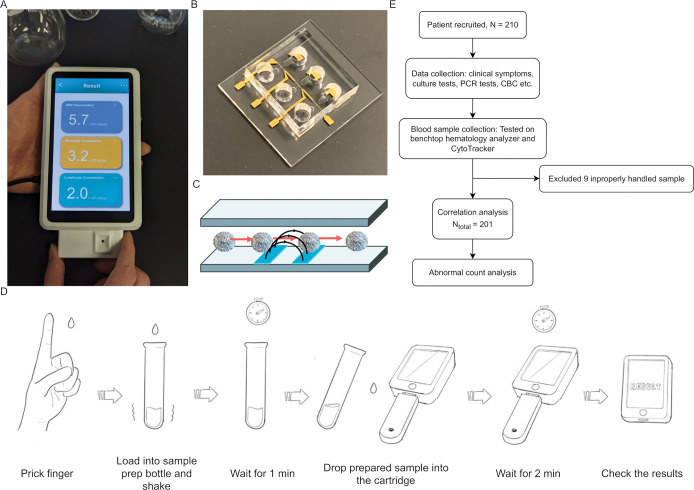
Fig 1. Study overview.
(A) Image of CytoTracker device prototype. (B) An image of the CytoTracker microfluidic impedance cytometer. (C) Schematic of sensing mechanism. (D) A diagram of the proposed user workflow. Drop of blood is obtained from patient, then placed into sample processing tube for lysis of red blood cells. After waiting for one minute, several drops of processed blood are squeezed into the test strip (plugged into device). After waiting for two minutes, the result is available for the user. (E) An overview of study workflow. 210 adult patients with symptoms were recruited. Nine patients were excluded due to the sample being improperly handled during shipment. As a result, the final analysis was obtained from 201 patients.