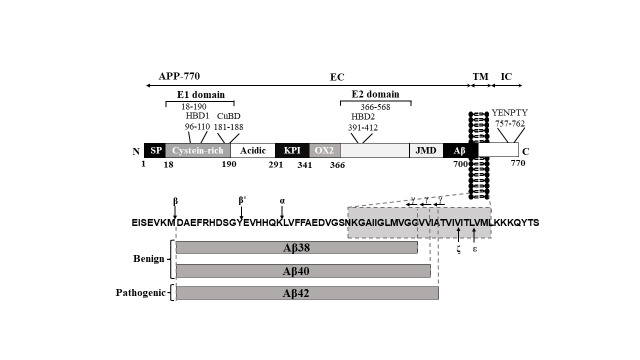
Figure 1.
Structure of APP: APP770 structure and Aβ peptide fragment. Protein structure (APP770). APP is composed of three domains: extracellular domain (EC), transmembrane domain (TM), and intracellular domain (IC). APP has an N-terminal signal peptide (SP); E1 domain with a heparin-binding domain (HBD1), a copper-binding domain (CuBD); acidic region; APP751 and APP770 contain a Kunitz protease inhibitor (KPI) domain and an Ox-2 antigen domain; E2 domain with a second heparin-binding domain (HBD2). BACE cleaves APP after Met671(β) and Tyr681(β′), whereas ADAM10 processes APP inside the Aβ peptide sequence after Lys687(α). γ-Secretase cleavage in the transmembrane region (TM) yields primarily 38, 40 and 42 amino acid residue-long Aβ peptides Aβ38, Aβ40 and Aβ42. ζ-site Aβ46 and ε-site Aβ49 downstream of the γ-site proximal to the membrane-intracellular boundary (for details concerning the γ-, ζ- and ε-sites).