Figure 4.
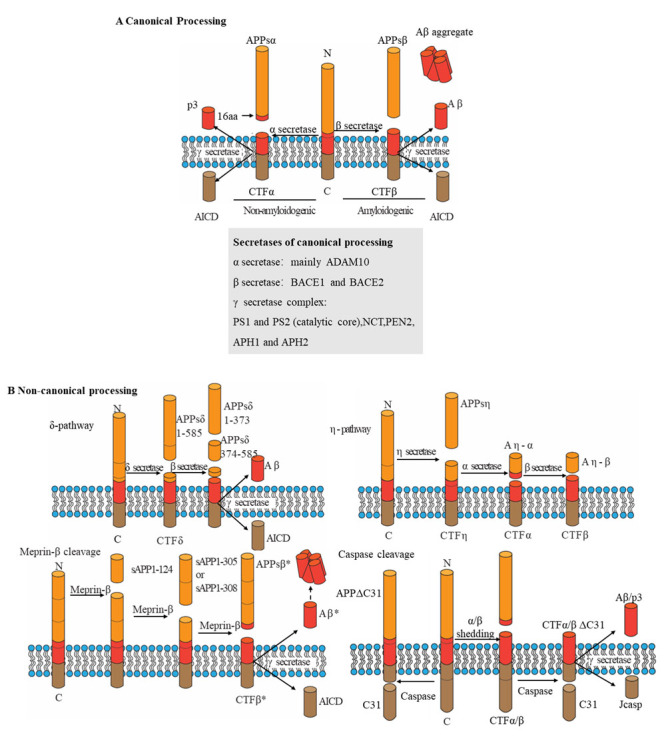
Schematic overview of APP-processing pathways. (A) The graphic displays conventional amyloid precursor protein manufacturing routes (APP). In the amyloid (Aβ) region (left), processing by secretase along the nonamyloidogenic pathway releases sAPPα (α-secretase-generated APP ectodomain fragment) and generates p3. The amyloidogenic pathway (right) produces Aβ (through β- and γ-secretase cleavage) and sAPPβ. An intracellular fragment (APP intracellular domain (AICD)) is released during both procedures. The locations of areas of cleavage are provided. B The graphic illustrates nonstandard APP processing. Secretase cleaves APPs into three soluble fragments and a C-terminal fragment-δ (CTFδ) that is subsequently processed by β- and γ secretase (top left panel). Three sites are utilized by Meprin β- and γ-secretase to form three soluble fragments (lower left panel). CTFβ* is one amino acid residue shorter than CTFβ and is generated after the release of sAPPβ*. Aβ* denotes the amino-terminally shortened version of Aβ2-x. By secretase cleavage, soluble APPsη and CTFη are produced, which are subsequently further processed by α-secretase or β-secretase to form Aηα or Aηβ (top right panel). Caspases cleave within the intracellular domain to form C31, which is then cleaved by secretases to yield Jcasp (lower right panel). (B) The diagram illustrates Aβ-generating and N-terminal cleavage sites. The numbers represent the C-terminal residues of full-length APP695 and the processing sites for APP. The upper panel depicts the positions of canonical secretases, with the residues targeted by γ-secretase numbered in relation to the Aβ N-terminal. The locations of noncanonical processing are depicted in the bottom panels. APH1, anterior pharynx defective 1; NCT, nicastrin; PEN2, presenilin enhancer 2; ADAM10, disintegrin and metalloproteinase domain-containing protein 10; PEN2, presenilin enhancer 2.
