Figure 2.
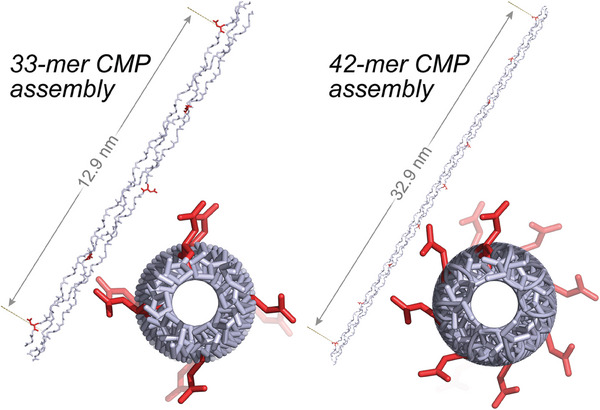
Comparison of molecular models for the symmetric self‐assembly of 33‐mer and 42‐mer CMPs into nanofibers. A single Asp residue is marked as red on each peptide in the assembly, and the orientation of this side chain is tracked along the assembly relative to the triple‐helical axis. The shortest distance between two such residues appearing on the same side of the triple‐helical assembly is reported. A top‐down view highlights differences in the distribution of Asp side chains around the triple helix. In contrast to one featuring a 42‐mer, the SESSA model of a 33‐residue CMP produces a regular arrangement of potential interaction partners along the triple‐helical axis and presents a geometry favorable for nanofiber association and bundling.
