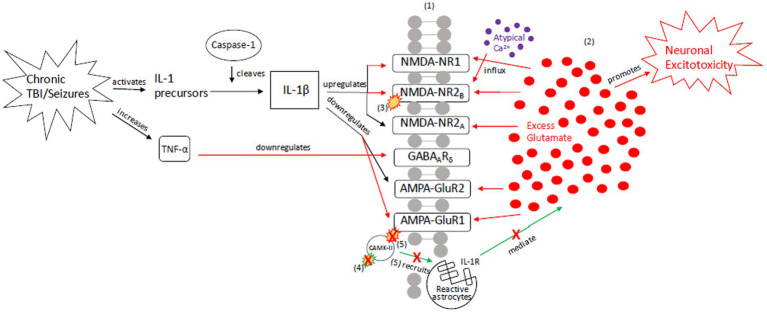
Figure 3.
Our proposed recurrent injury response (RIR) mechanism outlining reactive signaling to chronic and/or moderate–severe TBI and chronic and/or prolonged seizures. This mechanism shunts cellular signaling toward pro-death response pathways of apoptosis and necrosis due to imbalanced glutamate release and reuptake, Ca2+-driven ER stress responses, and apoptotic-necrotic dynamics. In response to chronic injury, caspase-1 cleaves IL-1 precursors, resulting in IL-1β formation, and TNF-α downregulates GABAA receptors (18, 108–113). However, unlike the AIR mechanism, IL-1β increases NMDA receptor activity via GluNR2B phosphorylation (112). Increased NMDA receptor densities contribute to atypical Ca2+ influx and prolonged excitotoxic signaling. Concurrently, AMPA-GluR1 and-GluR2 receptors are down-regulated in response to chronic injury, resulting in dysregulated CaMK-II autophosphorylation and AMPA-GluR1 site phosphorylation (21–23, 49, 114–116). Due to disrupted CaMK-II phosphorylation and autophosphorylation, reactive astrocytes cannot be successfully recruited to the synapse to clear excess glutamate and proteasome recruitment into dendritic spines is impaired, respectively (117). The result is neuronal excitotoxic depolarization and propagation, neurotoxic release of ATP, and preferential apoptotic signaling (118). (1) = Neuronal membrane, (2) = Synaptic cleft, (3) = IL-1β-activated NMDA-NR2B phosphorylation, (4) CaMK-II autophosphorylation, (5) CaMK-II-AMPA-GluR1 phosphorylation. Red = Excitotoxic signaling, Green = Neuroprotective signaling. X = response reduction/down-regulation.