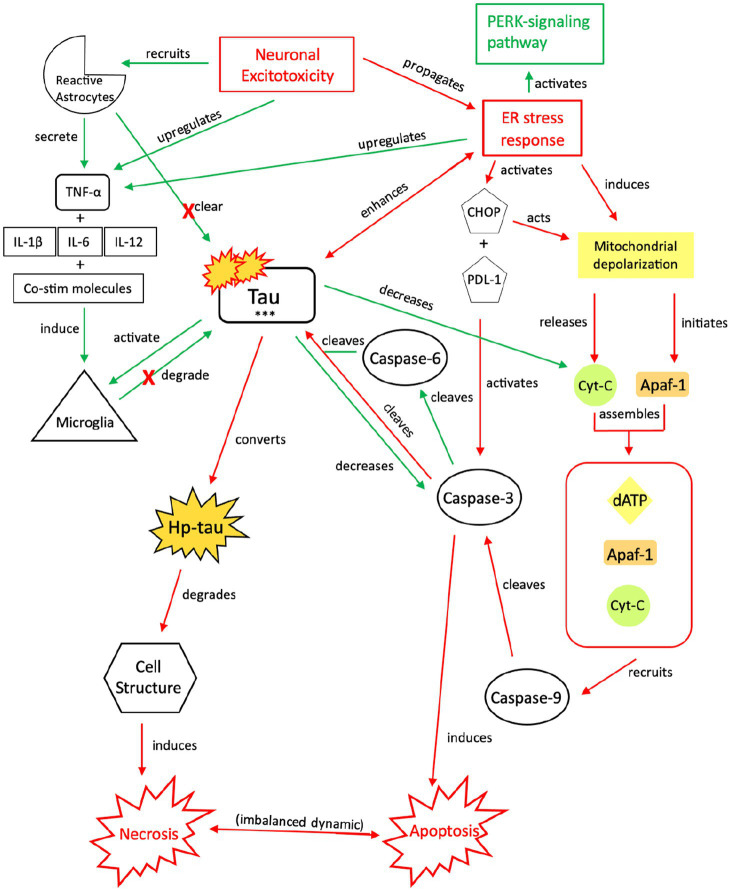
Figure 5.
Our proposed injurious response mechanism involving tau (NIT) outlining injurious tau signaling and the resulting imbalance in apoptotic-necrotic signaling due to a chronic or sustained injury response from TBI or seizures. Similar to the NPT response, neuronal excitotoxicity imbalances the ER stress response, which activates two pathways: the PERK pathway and pro-cell death signaling pathway. Increased presence of p-tau decreases the concentration of cyt-c and caspase-3, inhibiting apoptotic signaling; although downregulated, caspase-3 still cleaves tau and contributes to tau’s toxic effects, which further reverts the cell away from apoptotic signaling toward necrosis (119–121). To compensate for this shift, increased cytokine expression, increased TNF-α, and increased tau concentrations recruit reactive astrocytes and microglia to break down excess tau into non-toxic components (107, 131, 132). However, unsuccessful breakdown of tau by microglia and reactive astrocytes results in a build-up of toxic tau aggregates that are secreted extracellularly (137, 138). Adjacent cells attempt to break down the toxic tau into non-toxic components (99), but chronic activation of the NIT pathway due to recurrent or sustained injury dysregulates this response, resulting in an injurious build-up of toxic levels of tau, hp-tau, and NFTs, which reinforce necrotic signaling (139–141). PERK, protein kinase R-like ER kinase; TNF, tumor necrosis factor; IL, interleukin; Co-stim, co-stimulatory (molecules); cyt-c, cytochrome-c; Apaf-1, apoptotic peptidase activating factor-1; dATP, deoxyadenosine triphosphate; NFTs, neurofibrillary tangles; Red, Pro-death signaling; Green, Neuroprotective signaling; o-tau, tau oligomers; t-tau, total tau; p-tau, phosphorylated tau; ***, O-tau, t-tau, p-tau; X, response reduction/down-regulation.