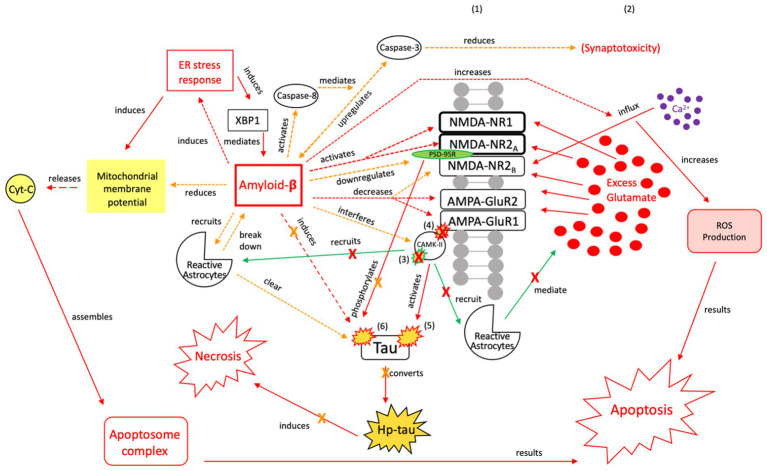
Figure 7.
The neuroprotective response of Aβ, aka the “last ditch effort” to revert the cell to pro-apoptotic signaling and reduce tau and Aβ toxicity. Selective NMDA regulation, downregulating scaffolding protein PSD-95, and activating caspase-8 reduce the excitotoxic effects of Aβ and atypical tau phosphorylation (154). PSD-95 receptor downregulation results in reduced tau phosphorylation and protection of synapses from the effects of Aβ, while caspase-8 activation indirectly reduces synaptotoxicity by mediating the relationship between Aβ and caspase-3 (163, 164). Aβ also reduces mitochrondrial membrane potential and directly induces the ER stress response, resulting in apoptosome complex formation and eventual ROS-induced apoptosis (165). Reactive astrocytes are recruited to break down Aβ and clear tau aggregates. However, Aβ also has injurious effects, as it increases intracellular Ca2+ and ROS production, while also acting directly on tau (154). Therefore, this mechanism is considered a “last ditch effort” to acutely kill the cell via apoptotic signaling to minimize the negative effects from toxic tau, Aβ accumulation, and necrotic signaling. We posit that limiting the effects of tau and Aβ toxicity is predicated on the acute nature of this response and treatment of the underlying pathology to avoid irreversible injury and/or a transition to a more widespread neurodegenerative process. XBP1, X-box binding protein 1; Cyt-c, cytochrome-c; ROS, reactive oxygen species; Red = Pro-death signaling, Green = Neuroprotective signaling, Orange = Aβ-involved signaling; X = reduction/down-regulation. Solid line = signaling cascade induced/propagated by the ER stress response and tau; dashed line = signaling cascades resulting from Aβ involvement. (1) = Neuronal membrane, (2) = Synaptic cleft, (3) = CaMK-II-autophosphorylation, (4) = CaMK-II-Glutamate receptor phosphorylation, (5) = CaMK-II-tau-phosphorylation, (6) = PSD95-NMDA receptor complex-tau phosphorylation.