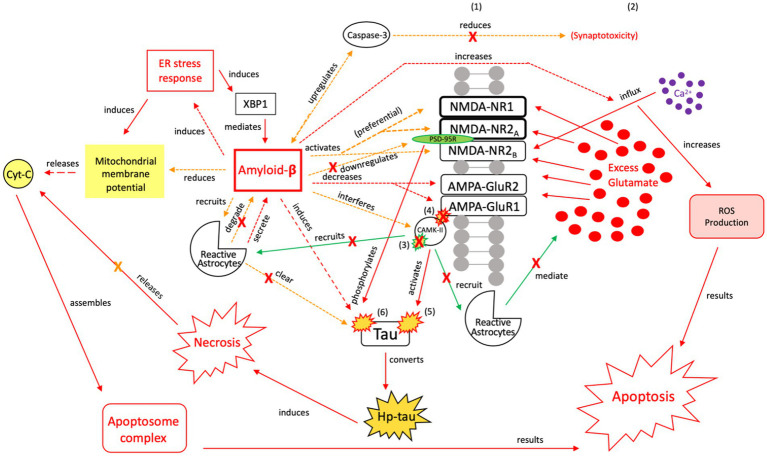
Figure 9.
The injurious response of Aβ, aka the failed “last ditch effort” to revert the cell to pro-apoptotic signaling and rebalance apoptosis-necrosis, due to recurrent or sustained Aβ signaling. Unlike neuroprotective Aβ responses, preferential activation of NMDA-R1 and-2A/B receptor subunits by Aβ (171, 172), and their increased surface expression regulated by PSD-95, adversely affects channel assembly and conductance (173), promoting further neuroexcitotoxicity, atypical tau phosphorylation, and increased susceptibility to Aβ (164). Unsuccessful toxic tau aggregate and Aβ breakdown by microglia [seen in (A)] propagates the injurious effects of Aβ-associated tau seeding and propagation (133). In the presence of dysregulated tau and Aβ mechanisms, as well as dysregulated microglial and reactive astrocytic clearance, the failure to reduce neuroinflammation and excitotoxic propagation results in a transition from neuroprotection to neurodegeneration. We posit that this point marks the transition from a injurious mechanism to a more widespread neurodegenerative process. XBP1, X-box binding protein 1; Cyt-c, cytochrome-c; ROS, reactive oxygen species; Red = Pro-death signaling, Green = Neuroprotective signaling, Orange = Aβ-involved signaling; X = reduction/down-regulation. Solid line = signaling cascade induced/propagated by the ER stress response and tau; dashed line = signaling cascades resulting from Aβ involvement. (1) = Neuronal membrane, (2) = Synaptic cleft, (3) = CaMK-II-autophosphorylation, (4) = CaMK-II-Glutamate receptor phosphorylation, (5) = CaMK-II-tau-phosphorylation, (6) = PSD95-NMDA receptor complex-tau phosphorylation.