Figure 4.
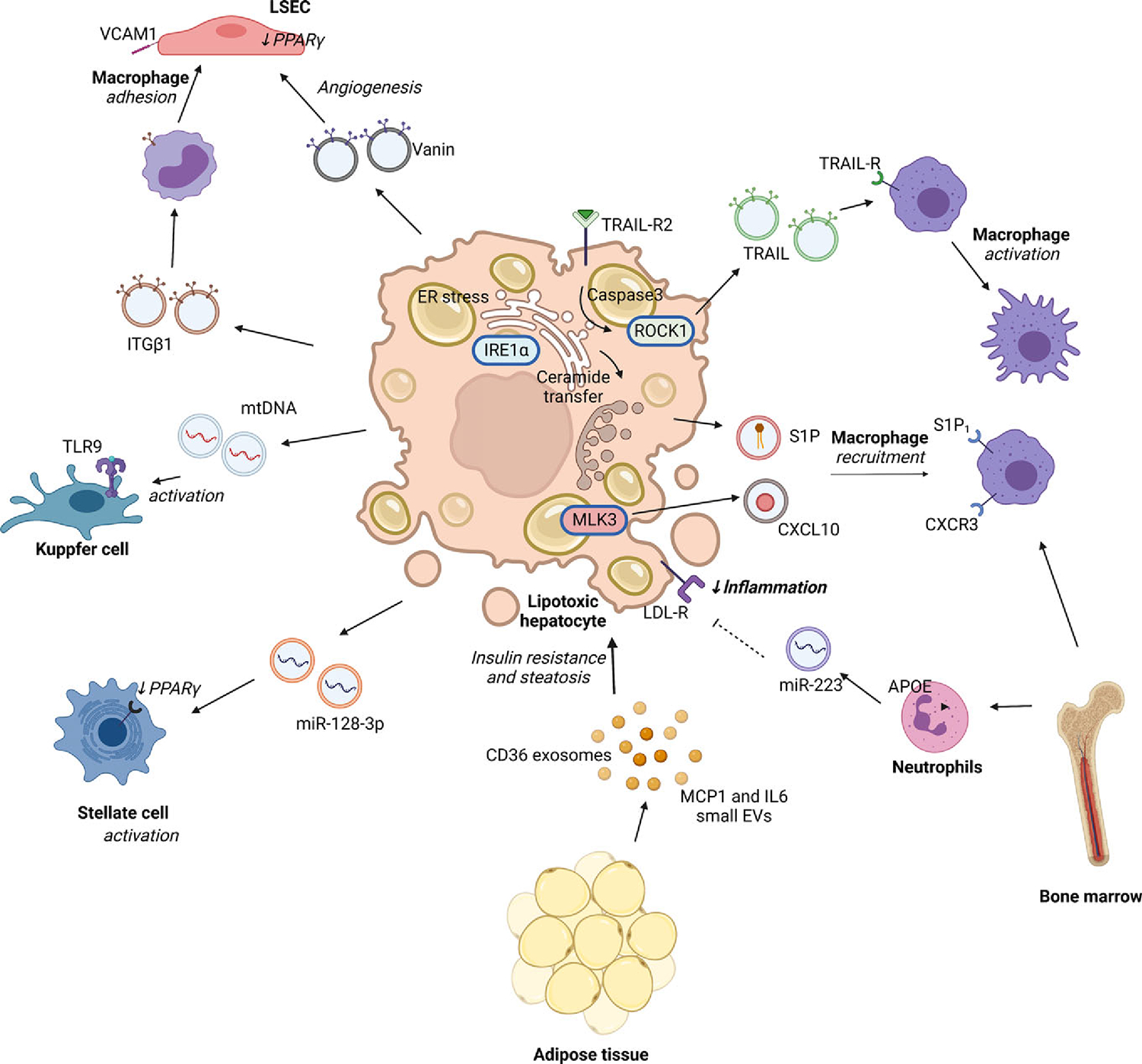
Role of extracellular vesicles in nonalcoholic steatohepatitis. EVs mediate multi-organ cross-talk—between the liver, adipose tissue, and bone marrow; as well as multi-cellular cross-talk—between hepatocytes, non-parenchymal cells in the liver, and immune cells. Lipotoxic hepatocytes activate intracellular stress responses including endoplasmic reticulum (ER) stress-mediated activation of inositol requiring enzyme 1 α (IRE1α), activation of serine threonine Rho- kinase 1 (ROCK1) and caspase 3, and mixed lineage kinase 3 (MLK3) which increase the formation and release of EVs. These EVs are heterogeneous and carry diverse cargo such as proteins including Vanin, TRAIL, CXCL10, nucleic acids including microRNAs (mir-128–3p, miR-223) and mitochondrial DNA (mtDNA), and lipids such as sphingosine 1 phosphate (S1P) which serve ligands for a multitude receptor-mediated or epigenetic regulatory signaling pathways in recipient cells. Though factors targeting subsets of EVs to specific recipient cells remain unknown, studies demonstrate that specific cell types respond to certain cargoes. For example, lipotoxic ER stress in hepatocytes can increase release of EVs containing ceramide-derived S1P which is a chemoattractant to proinflammatory circulating macrophages expressing S1P receptors. Lipotoxic EVs also contain CXC motif chemokine ligand 10 (CXCL10), TNFα-related apoptosis-inducing ligand (TRAIL), mtDNA which engages CXC motif chemokine receptor 3 (CXCR3), TRAIL-receptor (TRAIL-R), and Toll-like receptor 9 (TLR9), respectively. In contrast, hepatocyte uptake via the low-density lipoprotein receptor (LDL-R), of miR-223 enriched EVs from neutrophils may have an anti-inflammatory effect in NASH. Vanin-enriched hepatocyte-derived EVs and miR-128–3p enriched EVs increase hepatic stellate cell activation. Integrin beta 1 (ITGB1) in hepatocyte-derived EVs is internalized by circulating monocytes to increase their adhesion to liver sinusoidal endothelial cells (LSEC). Adipose tissue-derived EVs containing monocyte chemotactic protein 1 (MCP1), interleukin 6 (IL6), and cluster of differentiation 36 (CD36) influence hepatocyte insulin resistance and lipid metabolism. Cells and tissues are denoted in bold, and the biological effect is italicized.
