Figure 5.
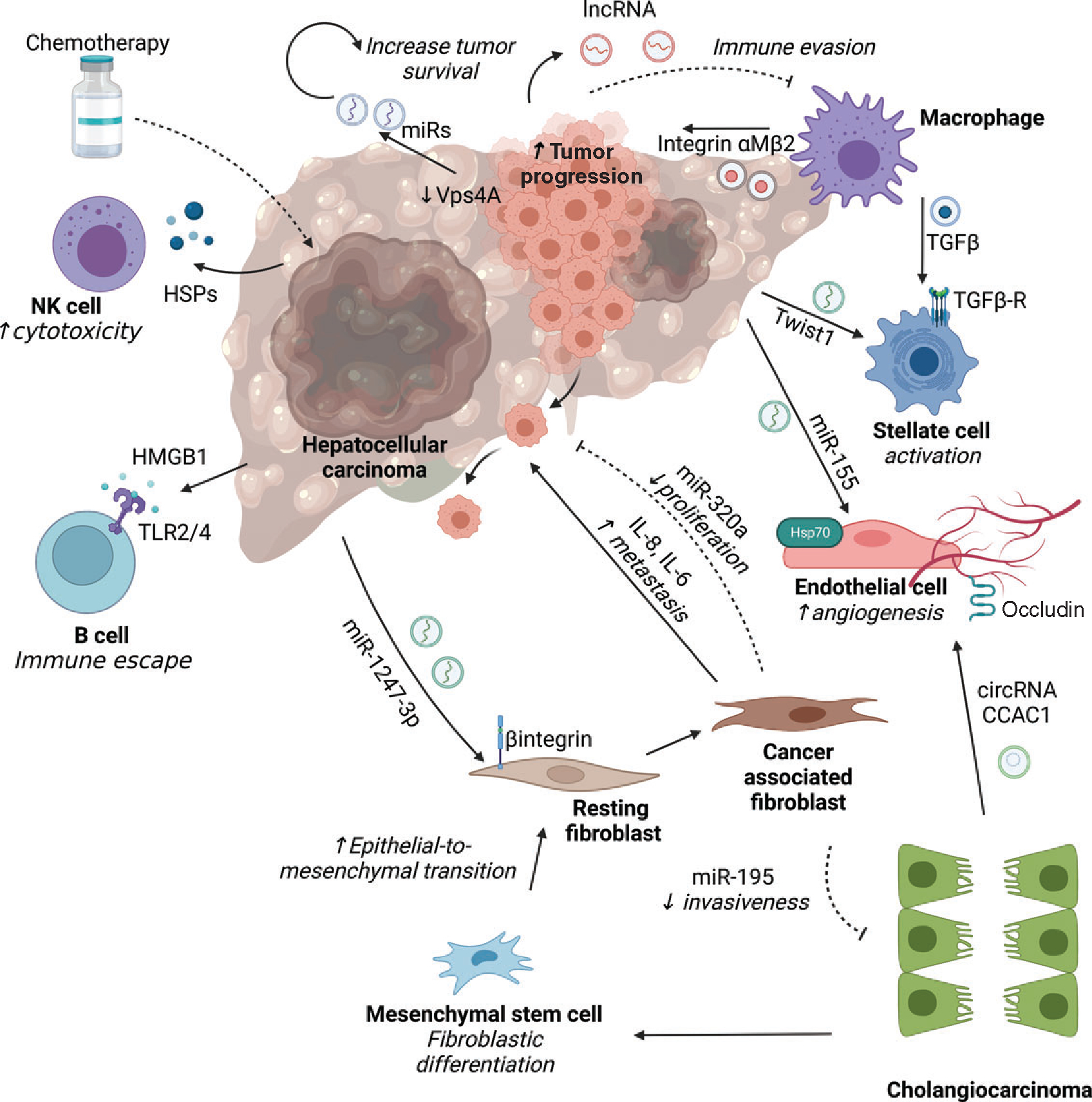
Role of extracellular vesicles in primary tumors of the liver. In hepatocellular carcinoma (HCC) as well as cholangiocarcinoma (CCA), EVs are implicated in cross-talk between cancer cells and other cell types inhabiting the tumor microenvironment. In HCC, tumor cells mediate a feed-forward loop with tumor cells by downregulating expression of the vacuolar protein sorting 4 homolog A (VPS4A) that promotes secretion of EVs with bioactive cargo such as microRNA (miRs) with oncogenic potential or long noncoding RNA (lincRNA-regulator of reprogramming) which confer chemoresistance. HCC-derived EVs mediate diverse processes such as angiogenesis via miR-155 containing EVs that activate heat shock proteins 70 on endothelial cells, epithelial-mesenchymal transition via miR-1247–3p that activate β-integrin on fibroblasts, and stellate cell activation via Twist1. Conversely, cancer-associated fibroblast (CAF) derived EVs carrying interleukins (IL6/8) promote HCC metastatic potential via tranglutaminase2 signaling, while miR-320a reduces tumor progression by suppressing PBX homeobox 3 (PBX3) signaling. Crosstalk with immune cells occurs via tumor-derived exosomes containing high mobility group box 1 (HMGB1) that mediate immune escape by binding to toll-like receptors 2/4 on B cells, and heat shock proteins that activate cytotoxic natural killer (NK) cells after exposure to chemotherapy. Tumor-associated macrophage-derived exosomes that contain integrinαMβ2 and TGFβ boost the migratory potential of HCC by activating matrix metalloproteinase-9 signaling and stellate cell activation respectively. In cholangiocarcinoma, EVs containing circular RNAs (circ-CCAC1) disrupt endothelial barrier integrity by downregulating intercellular junction proteins such as occludin and promote angiogenesis. CCA-EVs promote tumor stroma formation by inducing fibroblast differentiation of mesenchymal stem cells, and conversely CAF-derived EVs carrying miR-195 inhibit CCA growth. Cell types are denoted in bold, and the biologic effect is italicized.
