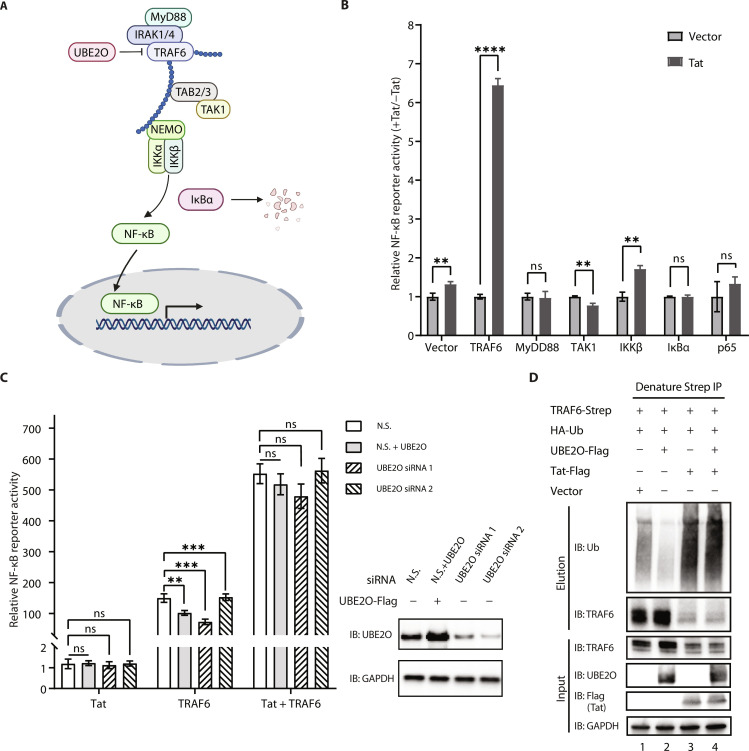
Fig. 1. Tat enhances TRAF6-dependent NF-κB activation in a UBE2O-independent manner.
(A) Schematic of TRAF6-mediated canonical NF-κB activation. (B) Identification of cellular signaling molecules related to NF-κB activation by HIV-1 Tat. Human embryonic kidney (HEK) 293T cells were transfected with an NF-κB luciferase plasmid reporter and the internal control NanoLuc luciferase (NLuc) as well as expression plasmid encoding each of the candidate proteins involved in the NF-κB signaling (MyD88, TRAF6, TAK1, IKKβ, IκBα, and p65), together with an empty vector or the plasmid encoding Tat for 24 hours. NF-κB activation was analyzed by using the Nano-Glo Dual-Luciferase Reporter Assay. (C) Left: NF-κB reporter luciferase activity was measured in HEK293T cells previously transfected for 48 hours with small interfering RNA (siRNA) against UBE2O (siUBE2O 1 or siUBE2O 2) or non-silencing (N.S.) siRNA and then cotransfected for 24 hours with an empty vector, Tat, TRAF6, or TRAF6 + Tat together with an NF-κB luciferase reporter and the internal control NLuc. Right: Western blot analysis of UBE2O protein expression. (D) In vivo ubiquitination of ectopically expressed Strep-tagged TRAF6 and hemagglutinin (HA)-ubiquitin in the presence or absence of Tat-Flag, UBE2O-Flag, or Tat-Flag/UBE2O-Flag. Cells were lysed under denaturing conditions, and IPs were performed with Strep-Tactin resin to detect ubiquitination by Western blot with an anti-ubiquitin antibody. The graphs in (B) show the means ± SD of three biological replicates and were normalized to cotransfected NLuc activities. The graphs in (C) show the means ± SD of four biological replicates and were normalized to cotransfected NLuc activities. ns P > 0.05, *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001, and statistical significance was assessed by a two-tailed unpaired Student’s t test.