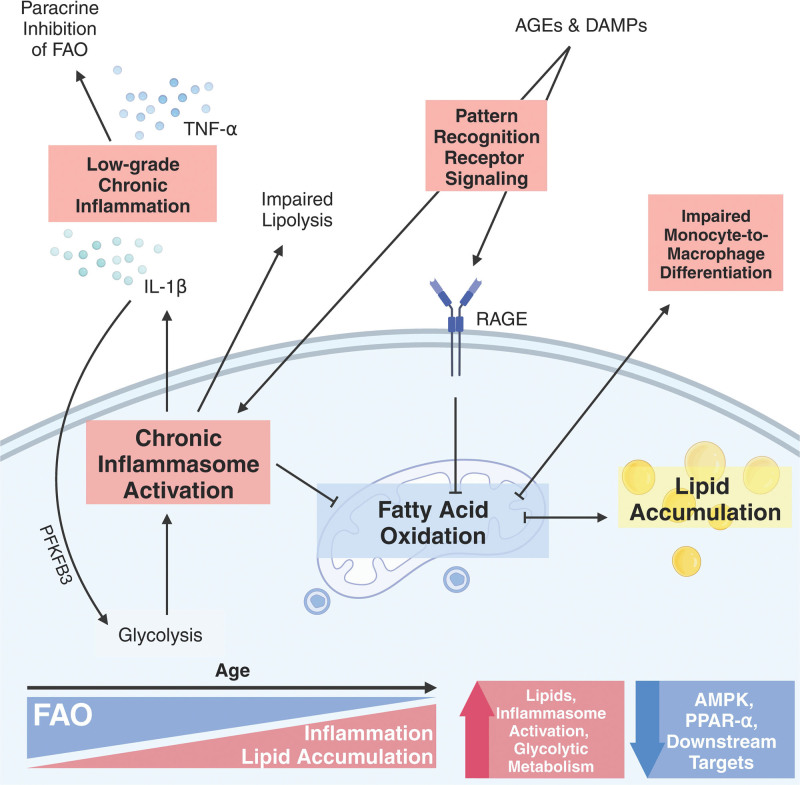
Figure 1.
Mechanism of fatty acid oxidation dysregulation during aging. This schematic gives a broad overview of the changes seen in macrophages and monocytes from older individuals. During aging, chronic stimulation by PAMPs and DAMPs causes the activation of pattern-recognition receptor pathways, like RAGE, TLRs, and the NLRP3 inflammasome. This increases pro-inflammatory cytokines like TNF-α and IL-1β and promotes the polarization of M1 macrophages that exhibit a pro-inflammatory phenotype. M1 macrophages also exhibit a robust shift towards glycolysis, which is supported by PFKFB3. These cytokines downregulate PPARα and FAO activation. Macrophages during aging also exhibit a downregulation of AMPK signaling, increased triglyceride synthesis, an accumulation of lipid droplets. Decreases in AMPK signaling leads to impaired monocyte-to-macrophage differentiation, reduced FAO, accumulating ROS, and amplified activation of the NLRP3 inflammasome. Arrows pointing directly outwards from a central point represent positive/feed-forward processes, whereas arrows branching off from an inhibition line denote processes that develop as a result of downregulated pathway (eg, FAO). The figure was created with Biorender.com. AMPK, 5’ AMP-activated protein kinase; FAO, fatty acid oxidation; IL-1β, interleukin-1 beta; NLRP3, NLR family pyrin domain containing 3; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3; PPAR-α, Peroxisome proliferator-activated receptor alpha; RAGE, receptor for advanced glycation end products; ROS, reactive oxygen species; TNF-α, tumor necrosis factor-alpha.