Figure 4.
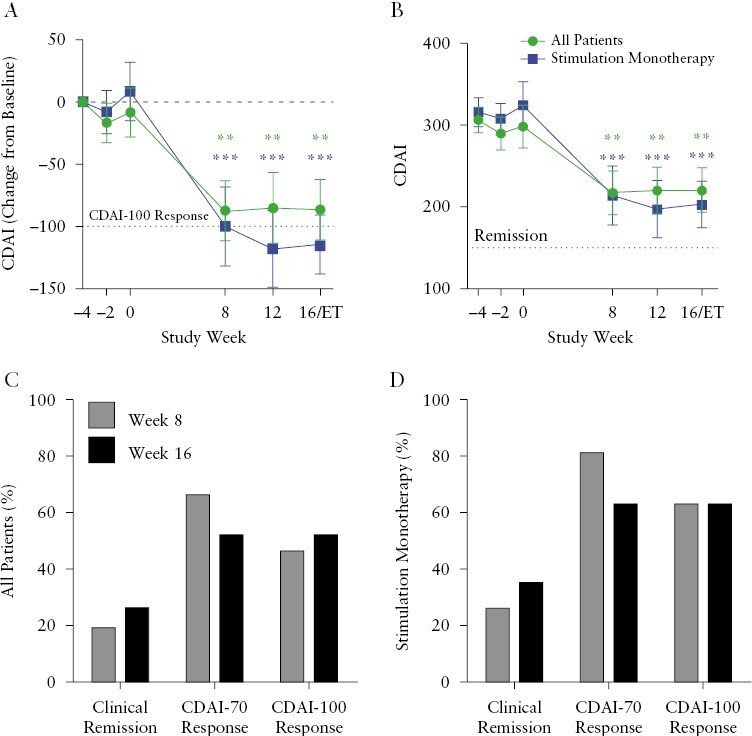
Clinical efficacy. [A] Change from baseline in CDAI [mean ± SEM] and [B] CDAI [mean ± SEM] over time in the All Patients Efficacy population [n = 16] and in the 12-patient Stimulation Monotherapy subpopulation. [C] Percent of All Patients or [D] Stimulation Monotherapy patients who achieved clinical remission [CDAI <150], CDAI-70 [CDAI decrease from baseline ≥70], and CDAI-100 [CDAI decrease from baseline ≥100]. CDAI and its change from baseline were analysed with a paired mixed-effects model [restricted maximum likeliness; REML] and adjusted with Bonferroni’s multiple comparisons test. **p <0.01, ***p <0.001. CDAI, Crohn’s Disease Activity Index; SEM, standard error of the mean.
