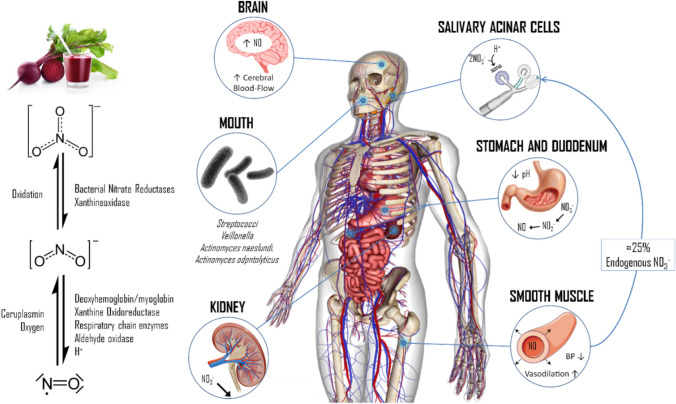
Fig. 1.
The nitrate/nitrite/nitric oxide (NO3−/NO2−/NO) pathway after dietary NO3− ingestion. Next to BR ingestion, oral microbiota on the posterior surface of the tongue is able to reduce NO3− to NO2− by means of their enzymatic machinery. The strict anaerobes Veillonella atypical and Veillonella dispar are the most important NO3− reducers; however, Actinomyces, Rothia, Prevotella, Neisseria, and Haermophilus are also present in the oral cavity. Even though this nonenzymatic reduction process continues in the stomach, where more NO2− and NO are produced due to the acid environment, a considerable amount of NO3− from blood (≈ 25%) is taken up by an electrogenic 2NO3−/H+ symporter called SLC17A5 (also known as sialin) in the salivary gland acinar cells [78]. Both dietary and saliva NO3−, and its reduced forms NO2− and NO, enter directly to systemic circulation after the absorption process in the stomach and intestine. Thus, the increase of NO3− and NO2− concentrations in blood allow the generation of NO by either enzymatic or non-enzymatic mechanisms (such as xanthine oxidoreductase, respiratory chain enzymes, aldehyde oxidase, methemoglobin formation, protons, etc.), especially under physiologic hypoxia and low pH [79]. Because of its short half-life (1–2 ms), once NO is produced in blood, it is broken down by hemoglobin or it can diffuse into the vascular smooth muscle cells or neurons and bind to guanylyl cyclase, which allows the allosteric activation of this last and subsequent cGMP production [80]. Here, cGMP acts as a second messenger and activates PKG, which in turn can modulate smooth muscle relaxation by several interlinked mechanisms: (i) activation of K+ channels leading to hyperpolarization; (ii) reduction of intracellular Ca2+ concentration; and (iii) activation of the myosin light-chain phosphatase [81]. Finally, NO3− is normally excreted in the urine by the kidneys. BP blood pressure, NO nitric oxide. Modified with permission from Bonilla et al. [4]