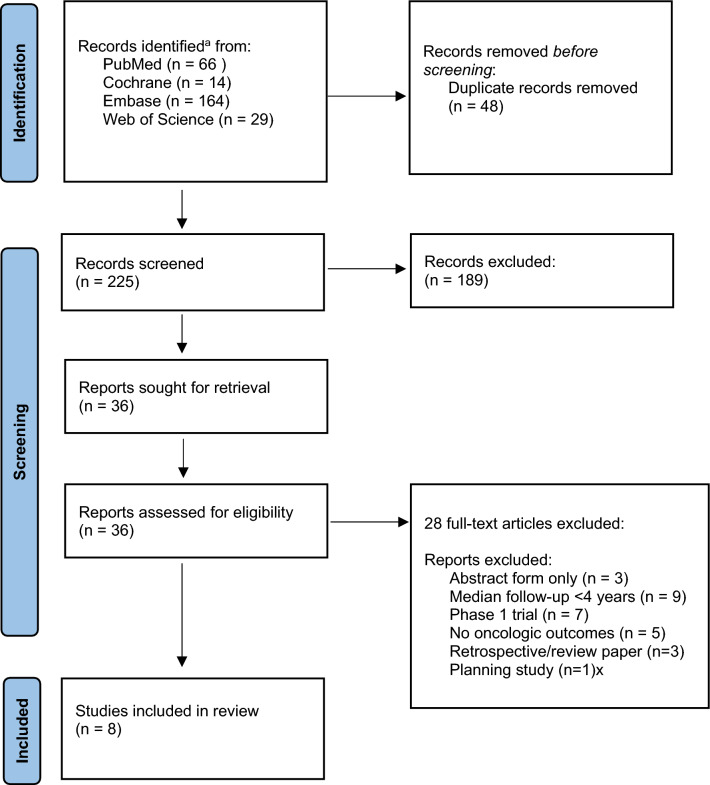
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. aSearch Strategy: (((prostate cancer) or (prostate)) AND ((radiation therapy) OR (radiotherapy) OR (focal boost) OR (boost) NOT (brachytherapy)) AND ((intraprostatic lesion) OR (intraprostatic nodule) OR (dominant) OR (IPL) OR (IPN) OR (DIL)) AND ((Phase I Clinical Trial) OR (Phase II Clinical Trial) OR (Phase III Clinical Trial) OR (Phase IV Clinical Trial) OR (Controlled Clinical Trial) OR (Multicenter Study) OR (Randomized Controlled Trial) OR (Pragmatic Clinical Trial) OR (Comparative Study)))