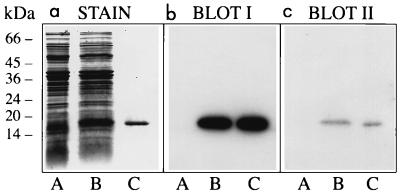
FIG. 3.
Binding of MHC-I molecules and β2m to curlin subunits. Three identical SDS-polyacrylamide gels were loaded with proteins solubilized from curli-expressing YMel bacteria by boiling in SDS-PAGE sample buffer (lane A). The same material with the addition of purified curlin subunits (approximately 1 μg) was separated in lane B. Curlin subunits alone were run in lane C. One of the gels was stained with Coomassie blue (a), whereas the proteins of the other two were transferred to Immobilon membranes and probed with 125I-MHC-I (b) or 125I-β2m (c). Molecular mass markers are indicated to the left.