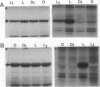
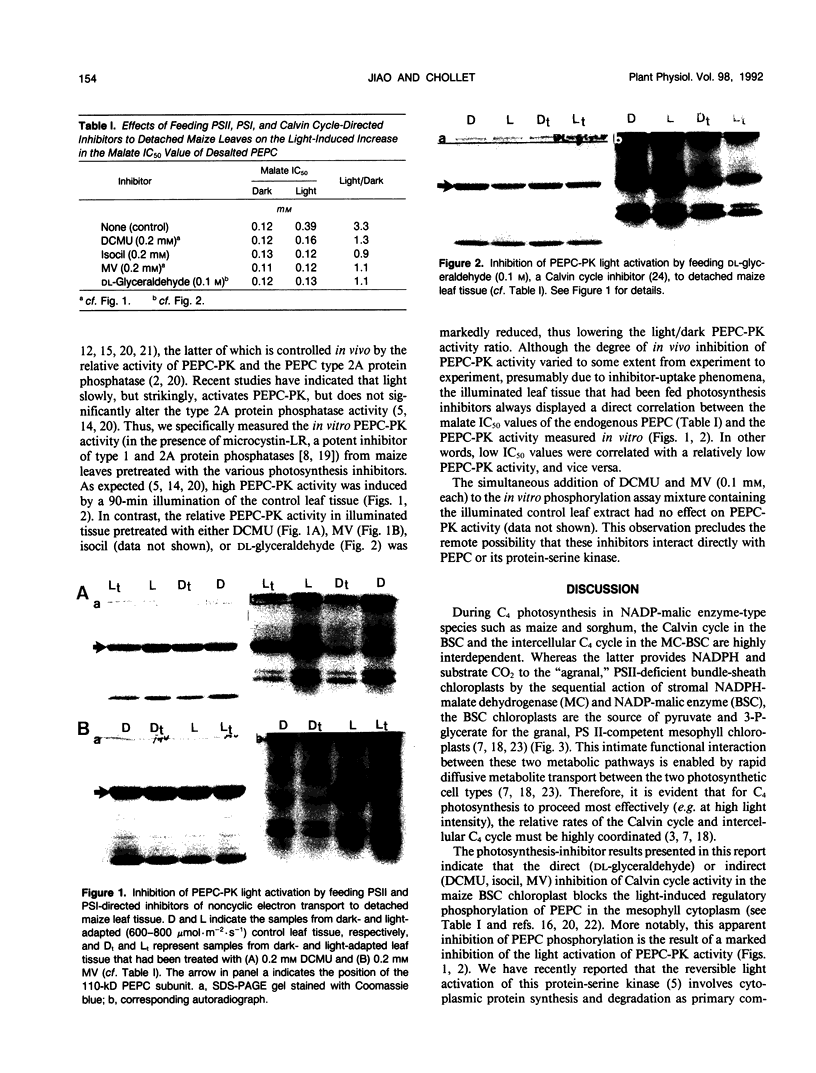
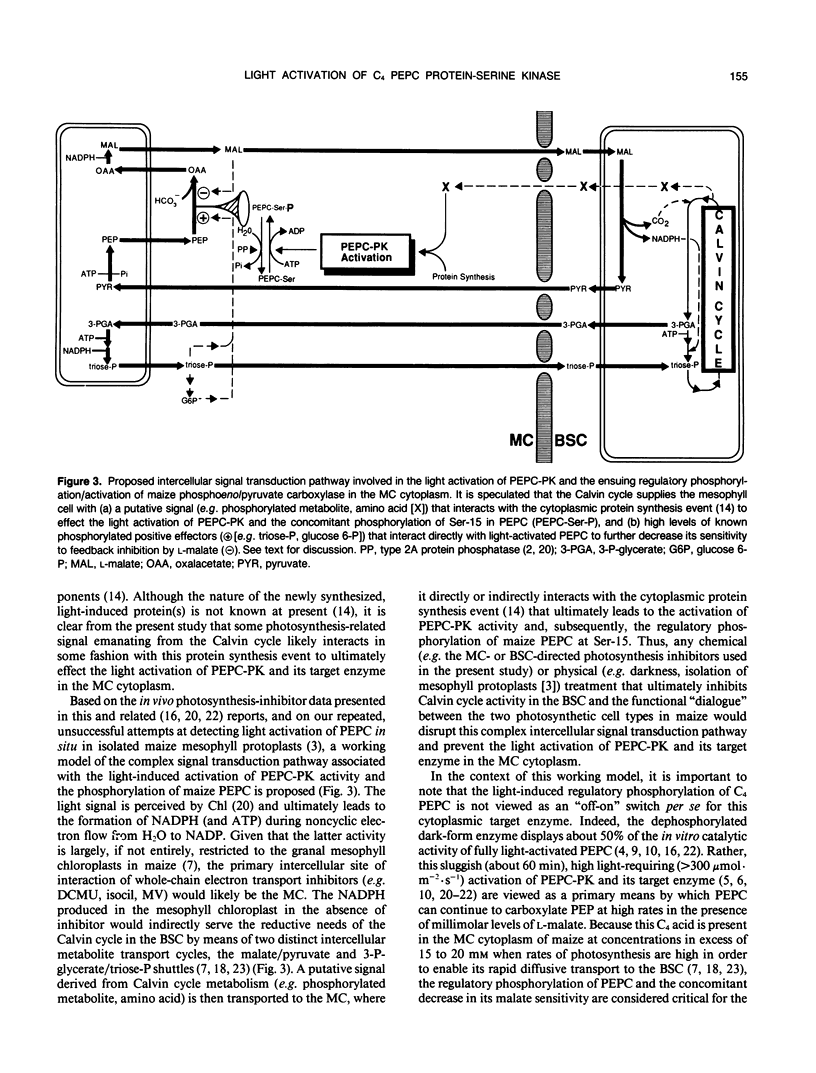
Abstract
C4 phosphoenolpyruvate carboxylase (PEPC) is post-translationally regulated by reversible phosphorylation of a specific N-terminal seryl residue in response to light/dark transitions of the parent leaf tissue. The protein-serine kinase (PEPC-PK) that phosphorylates/activates this mesophyll-cytoplasm target enzyme is slowly, but strikingly, activated by high light and inactivated in darkness in vivo by a mechanism involving cytoplasmic protein synthesis/degradation as a primary component. In this report, evidence is presented indicating that the inhibition of Calvin cycle activity by a variety of mesophyll (3-(3,4-dichlorophenyl)-1,1-dimethylurea, isocil, methyl viologen) and bundle sheath (dl-glyceraldehyde)-directed photosynthesis inhibitors blocks the light activation of maize (Zea mays L.) PEPC-PK and the ensuing regulatory phosphorylation of its target enzyme in vivo. Based on these and related observations, we propose that the Calvin cycle supplies the C4 mesophyll cell with (a) a putative signal (e.g. phosphorylated metabolite, amino acid) that interacts with the cytoplasmic protein synthesis event to effect the light activation of PEPC-PK and the concomitant phosphorylation of PEPC, and (b) high levels of known positive effectors (e.g. triose-phosphate, glucose-6-phosphate) that interact directly with the carboxylase. The combined result of this complex regulatory cascade is to effectively desensitize PEPC to feedback inhibition by the millimolar levels of l-malate required for rapid diffusive transport to the bundle sheath during high rates of C4 photosynthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Doncaster H. D., Leegood R. C. Regulation of phosphoenolpyruvate carboxylase activity in maize leaves. Plant Physiol. 1987 May;84(1):82–87. doi: 10.1104/pp.84.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarría C., Vidal J., Jiao J. A., Chollet R. Reversible light activation of the phosphoenolpyruvate carboxylase protein-serine kinase in maize leaves. FEBS Lett. 1990 Nov 26;275(1-2):25–28. doi: 10.1016/0014-5793(90)81430-v. [DOI] [PubMed] [Google Scholar]
- Honkanen R. E., Zwiller J., Moore R. E., Daily S. L., Khatra B. S., Dukelow M., Boynton A. L. Characterization of microcystin-LR, a potent inhibitor of type 1 and type 2A protein phosphatases. J Biol Chem. 1990 Nov 15;265(32):19401–19404. [PubMed] [Google Scholar]
- Huber S. C., Sugiyama T., Akazawa T. Light modulation of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol. 1986 Oct;82(2):550–554. doi: 10.1104/pp.82.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J. A., Chollet R. Light/dark regulation of maize leaf phosphoenolpyruvate carboxylase by in vivo phosphorylation. Arch Biochem Biophys. 1988 Mar;261(2):409–417. doi: 10.1016/0003-9861(88)90357-8. [DOI] [PubMed] [Google Scholar]
- Jiao J. A., Chollet R. Posttranslational regulation of phosphoenolpyruvate carboxylase in c(4) and crassulacean Acid metabolism plants. Plant Physiol. 1991 Apr;95(4):981–985. doi: 10.1104/pp.95.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J. A., Chollet R. Regulatory phosphorylation of serine-15 in maize phosphoenolpyruvate carboxylase by a C4-leaf protein-serine kinase. Arch Biochem Biophys. 1990 Dec;283(2):300–305. doi: 10.1016/0003-9861(90)90646-g. [DOI] [PubMed] [Google Scholar]
- Jiao J. A., Chollet R. Regulatory seryl-phosphorylation of C4 phosphoenolpyruvate carboxylase by a soluble protein kinase from maize leaves. Arch Biochem Biophys. 1989 Mar;269(2):526–535. doi: 10.1016/0003-9861(89)90136-7. [DOI] [PubMed] [Google Scholar]
- Jiao J. A., Vidal J., Echevarría C., Chollet R. In vivo regulatory phosphorylation site in c(4)-leaf phosphoenolpyruvate carboxylase from maize and sorghum. Plant Physiol. 1991 May;96(1):297–301. doi: 10.1104/pp.96.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J., Echevarría C., Vidal J., Chollet R. Protein turnover as a component in the light/dark regulation of phosphoenolpyruvate carboxylase protein-serine kinase activity in C4 plants. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2712–2715. doi: 10.1073/pnas.88.7.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabourniotis G., Manetas Y., Gavalas N. A. Photoregulation of Phosphoenolpyruvate Carboxylase in Salsola soda L. and Other C(4) Plants. Plant Physiol. 1983 Nov;73(3):735–739. doi: 10.1104/pp.73.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacKintosh C., Beattie K. A., Klumpp S., Cohen P., Codd G. A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990 May 21;264(2):187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- McNaughton G. A., MacKintosh C., Fewson C. A., Wilkins M. B., Nimmo H. G. Illumination increases the phosphorylation state of maize leaf phosphoenolpyruvate carboxylase by causing an increase in the activity of a protein kinase. Biochim Biophys Acta. 1991 Jul 10;1093(2-3):189–195. doi: 10.1016/0167-4889(91)90122-e. [DOI] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Photosynthesis by isolated chloroplasts. Inhibition by DL-glyceraldehyde of carbon dioxide assimilation. Biochem J. 1972 Aug;128(5):1147–1157. doi: 10.1042/bj1281147. [DOI] [PMC free article] [PubMed] [Google Scholar]