Abstract
Background
Patient accidental falls in a hospital environment are a serious problem for patient safety, and for the additional costs due to associated medical interventions.
Objective
The endpoints of this study were the assessment of the fall incidence in the hospital before and after the implementation of a multidisciplinary care-bundle, along with a cost-effectiveness evaluation.
Design
A stepped-wedge trial was conducted between April 2015 and December 2016 in Bologna University Hospital.
Methods
Incidence rates (IRs) of falls in both the control and intervention periods were calculated. A multilevel mixed-effects generalised linear model with logit link function, adjusted for age, sex, cluster cross-over timing and patients’ clinical severity was used to estimate odds ratios (OR) of fall risk of patients of the intervention group respect to the controls.
Intervention costs associated with the introduction of the care-bundle intervention were spread between patients per cluster-period-group of exposure. Incremental cost-effectiveness ratio was evaluated using total costs in the intervention and control groups.
Results
IRs of falls in control and intervention periods were respectively 3.15 and 2.58 for 1,000 bed-days. After adjustment, the subjects receiving the intervention had a statistically significant reduced risk of falling with respect to those who did not (OR = 0.71, 95% confidence interval: 0.60–0.84). According to the cost-effectiveness analysis, the incremental cost per fall prevented was €873.92 considering all costs, and €1644.45 excluding costs related falls.
Conclusions
Care-bundle had a protective effect on patients, with a statistically significant reduction of the fall risk. This type of intervention appears cost-effective compared to routine practices.
Keywords: falls in hospital, cost-effectiveness analysis, stepped-wedge design, care-bundle intervention, older people
Key Points
The stepped-wedge design proved to be suitable to evaluate the effectiveness of the new care-bundle.
The proposed care-bundle intervention showed to be cost-effective.
A reduction in fall risk was observed after the delivery of the care-bundle intervention
Introduction
Hospital-acquired falls (HAFs) constitute a growing concern for healthcare systems, and their incidence is increasing due to population ageing [1]. In 2017, the USA reported an average of 714 falls and 170 fall-related injuries per 1,000 older adults, accounting, respectively, for 35.6 million falls and 8.4 million fall-related injuries. In 2015, the USA overall medical costs related to HAF accounted for more than $50 billion [2]. In the UK, the incidence of falls in older adults (70+ years) was 12,099 per 100,000 persons. Meanwhile, the total burden of disease due to fall-related injuries in older adults in Western Europe has been estimated to be 1.4 million disability-adjusted life years [3].
Around 30% of HAF can result in fall-related traumatic injuries, and previous studies have shown that HAF can result in impaired cognitive and functional status as well as reduced physical performance and psychological well-being [4]. HAF’s related injuries are cause of considerable strain for the sustainability of healthcare systems, negatively impacting patient recovery and increasing the financial burden of healthcare institutions due to the increased length of hospital stay and higher rates of admission to long-term care facilities [5].
HAFs are the result of an interplay of multiple elements [6], both intrinsic, such as older age, and extrinsic, such as the hospital environment, inadequate staff communication, incomplete care planning and delayed care [6].
The most recent guidelines on HAF prevention suggest that a multifactorial perspective, involving cooperation between healthcare professionals (HPCs) [7], and patient/caregiver education [8, 9] may constitute the best approach for prevention. A recent meta-analysis [4], examining fall prevention methods in hospitals, showed strong evidence regarding the effectiveness of strategies targeting HPCs’ education, compared to low/very low effectiveness for assistive devices, additional rehabilitation therapies and environmental modifications. There is still uncertainty about the effectiveness of interventions aimed at reducing HAF, which suggests the need for further large-scale trials to provide robustness and generalisability of the evidence.
Moreover, only a few studies have considered the clinical effectiveness of the proposed interventions with their economic analysis.
The main objectives of the present study were: (i) to assess the effectiveness of a fall prevention care-bundle, with the introduction of a set of evidence-based practices, on HAF incidence rates (IRs) in older in-patients and (ii) to evaluate the cost-effectiveness of the care-bundle intervention.
Methods
A stepped-wedge trial was conducted between April 2015 and December 2016 involving 12 hospital units (geriatrics, internal medicine, rehabilitation) of the Bologna Local Health Authority and University Hospital. This article is reported according to the Consolidated Standards of Reporting Trials (https://www.equator-network.org/).
All patients aged 75 years or older consecutively admitted to the units, with or without a risk of falls, and patients younger than 75 years old with an ascertained risk of falls (Conley scale) were asked to participate in the study. According to the last epidemiological data from Europe and the USA regarding accidental falls in older adults, it was decided to include as a threshold all patients over 75 years (or younger if at risk of falls) due to the evident increase in the incidence of falls and severe fall-related injuries in the group ≥75 [2, 3].
Randomisation
In this stepped-wedge design, 12 hospital units (clusters) were grouped in four groups (3 clusters for each group) that received the intervention in a randomised order. The randomisation list for the groups was created with Stata 14.0 (www.stata.com). Three groups switched from control to intervention in a mono-directional crossover mode at three fixed time-points every 5 months. Each group was first assigned to usual care for 5 months and then switched to the care-bundle program for the remaining time of the survey. The first group (Group 1) introduced the intervention at baseline. The intervention was fully implemented at the end of the trial (fourth step) when all 12 clusters were allocated to the intervention (Figure 1).
Figure 1.
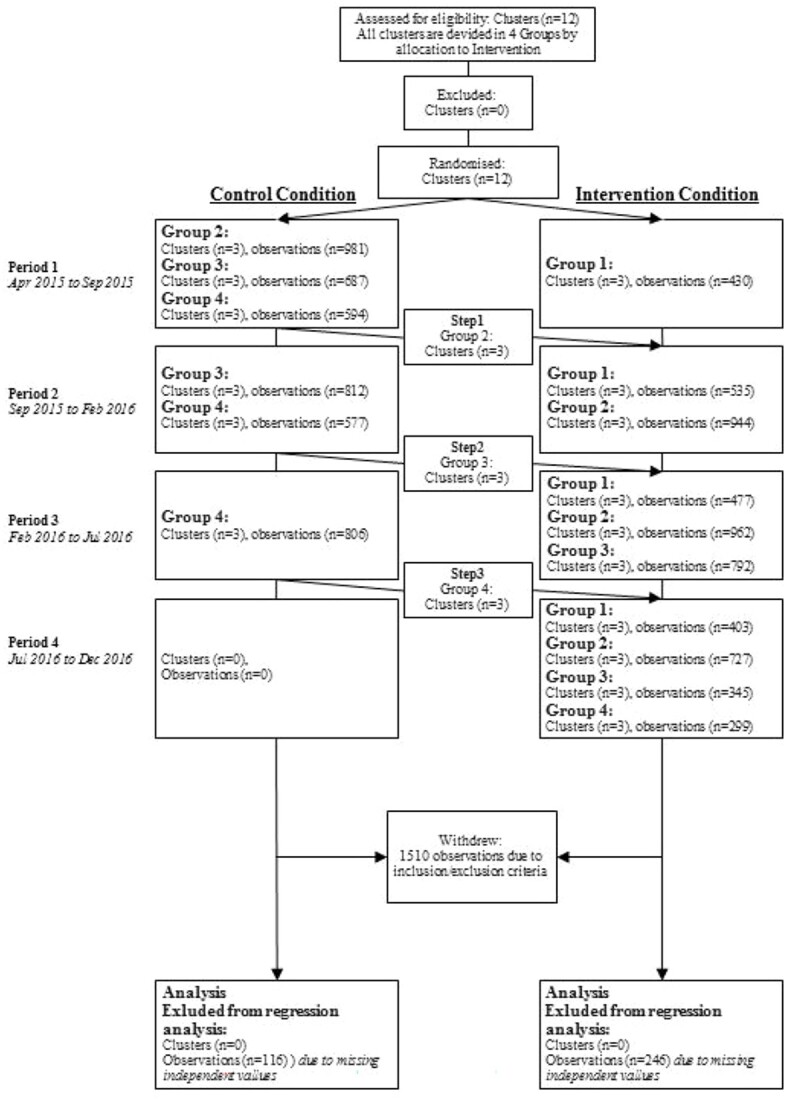
Cluster flow of the stepped-wedge study. Each group consisted of three clusters.
Given the nature of the intervention, blinding HCPs and patients was impossible.
Intervention
The following variables were collected at baseline: age, gender, degree of dependence on Activity of Daily Living on the Barthel Index [10], use of medications acting upon central nervous system and cardiovascular system (yes/no), orthostatic hypotension during a position change (yes/no), and length of stay (LOS). The care-bundle has been fully applied in the course of the selected introduction periods. Its components were:
Assessment of patients’ risk of falls at hospital admission, as measured with the Conley Scale [11];
Use of alert signs on top of bed frames to increase staff awareness of patients at high fall risk;
Systematic communication with patients and family members/caregivers regarding fall risk. Promotion of an open-door ward policy with the presence of informal caregivers;
Application of universal strategies to ensure environmental safety, such as checking and maintaining the integrity/functioning of night bells and lights, supplemental lighting, keeping floor surfaces clean and dry, etc.;
Conduction of rounds every 2 h to assess patients’ needs, such as go to the bathroom, change position or drink;
Regular re-assessment of the patient’s medications, particularly those that may affect the central nervous and cardiovascular system.
During control periods, patients received usual care, consisting of, for example, ensuring the functioning of bells/night lights, mobilisation aids, beds’ braking devices, etc.; keeping the patient’s bell/personal belongings within reach and the bed in the lowest position; and using the right size closed footwear with non-slippery soles.
In case of falls, each incident was recorded on the Incident Reporting System of each hospital electronic healthcare records (EHR). HPCs were asked to specify if the patient was found on the ground, or supported during the fall and ‘accompanied to the ground’. Incidence data were retrieved from the EHRs of the hospitals involved.
The training included a meeting with the HPCs involved in the study, which covered an explanation of the study aim, the presentation of the data collection tools and a focus on the rationale of the intervention, along with the clinical practice activities that would be monitored.
The collection of data on care-bundle-related practices was considered a trigger for regularising behaviour. Two research fellows were recruited for the conduction of the study. They periodically visited the units to collect the Case Report Forms and support the staff.
Primary endpoint
The primary endpoint was the incidence of HAF measured during the intervention and the control period.
Secondary endpoints
Secondary endpoints were the costs: (a) resulting from the care-bundle introduction, including costs for staff training, time and personnel resources, equipment and other materials and administrative expenses; (b) resulting from falls and fall-related injuries, such as diagnostic procedures, specialist visits, treatments (e.g. surgery) and LOS.
Ethics
The study followed the principles of the Declaration of Helsinki and was approved by the Ethical Committee of Bologna University Hospital. The registration number of the study is NCT03147521. Written consent was obtained from patients or their legal representatives for patients with cognitive impairment. Patients could withdraw from the study at any time without negative repercussions on their care.
Sample size
The study involved 12 hospital units with approximately 15,500 admissions on average (Hospital data 2011 and 2012) and about 158,000 days of hospitalisation and a rate of average falls of 4 × 1,000 bed-days of hospitalisation. Assuming a decrease in the incidence of falls from a rate of 4 × 1,000 bed-days to 2 × 1,000 bed-days, with a sample size of 30,000 admissions per 2 years, the test power in a stepped-wedge design was over 95%, with an alpha error of 0.05. The power was estimated using the correction formula for the sample size of the stepped-wedge design [12] with an intra-class correlation coefficient of 0.10, by NCSS-PASS 2014 (www.ncss.com). The fall rate estimates and the intra-class correlation coefficient were obtained from 2012 data.
Statistical analysis
Stata 14.0 was used to conduct the statistical analyses.
Descriptive statistics were calculated: median and inter-quartile ranges for continuous variables and counts and percentages for categorical ones.
HAF IRs in control and intervention periods were calculated as the ratio between the number of falls and the number of person-days of hospitalisation. Overall crude Incidence Rate Ratio (IRR) was calculated with its 95% confidence interval (95%CI). A multilevel mixed-effects generalised linear model (MEGLM) with Bernoulli distribution, logit link function and robust variance estimation adjusted for age, sex, timing of cluster cross-over and variables concerning clinical severity of patients was used to estimate the HAF adjusted odds ratios (OR) for those who received the intervention compared to the others (OR, 95%CI).
Economic evaluation
The economic evaluation adopted the perspective of the health system. For each of the patients, the costs of procedures (e.g. computed tomography [CT], radiography, surgery, etc.) were recorded. Unit costs of each procedure were taken from the list of tariffs of the Regional Health System of Emilia-Romagna (year 2016). Moreover, a cost for the LOS was computed for each patient, based on the length of hospitalisation and an estimated average cost per day. This estimate was obtained adopting a micro-costing approach, and it was averaged over hospital units.
The surgeries performed as a consequence to the falls were: closed reduction of shoulder dislocation (unit cost €51) and aesthetic suture of face wounds (€34.05) in the intervention group (IG); partial hip replacement (€12636.87) and closed reduction of femur fracture with internal fixation (€10656.14) in the control group (CG). Costs at the patient level were estimated using prices from LOS in a hospital unit (€220.45 per day referring to year 2015). Other procedure total costs per patient were: €44.4 (16–508.2) median (min–max) in the CG and €83.15 (19–498.9) in the IG. The most frequent procedure was a head CT scan (unit cost €83.15). The total procedure costs per patient were calculated as the sum over procedures of the unit cost times the number of procedures received. Costs were not discounted due to the short time horizon of the study. Moreover, this allowed us to ensure consistency between the way we treated costs and effectiveness with respect to discounting, given that our measure of effectiveness (IR) could not be discounted [13].
Costs related to the implementation of the care-bundle program, relevant only for the IG, were calculated per cluster, period of intervention and group of exposure. These costs included: staff training, printing materials and aids (walkers, folding self-propelling/tilting wheelchairs). To account for differences in exposure across patients, total costs per patient were converted into costs per day.
We performed a cost-effectiveness analysis, using IRs as an (inverse) measure of effectiveness. It was not possible to undertake a cost-utility analysis, due to the lack of data on quality of life. A typical advantage of cost-utility analysis is that it makes it easier to conclude whether the intervention is good value for money. Nonetheless, in the Discussion, we consider the results of our cost-effectiveness analysis using this perspective, considering the relevant literature.
The incremental cost-effectiveness ratio (ICER) was defined as:
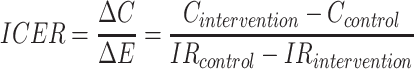 |
(1) |
where 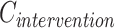 and
and 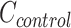 are the average costs per day, respectively, in the intervention and CG.
are the average costs per day, respectively, in the intervention and CG. 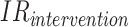 and
and 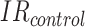 are the IRs (number of falls per 1,000 bed-days) in the two groups. Note that, unlike in the standard definition of the ICER, we subtract effectiveness of the IG from that of the CG in the denominator, because the higher the
are the IRs (number of falls per 1,000 bed-days) in the two groups. Note that, unlike in the standard definition of the ICER, we subtract effectiveness of the IG from that of the CG in the denominator, because the higher the 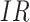 , the lower the effectiveness. This allowed us to interpret the sign and the magnitude of the ICER in the standard way. The ICER computed according to Equation (1) can be interpreted as the cost of preventing one fall.
, the lower the effectiveness. This allowed us to interpret the sign and the magnitude of the ICER in the standard way. The ICER computed according to Equation (1) can be interpreted as the cost of preventing one fall.
Results
In the 2-year study period 11,881 subjects were screened for participation, and 10,371 met the inclusion criteria and were enrolled in the study. Figure 1 shows the study flowchart, and Table 1 shows the patients’ baseline characteristics by hospital unit.
Table 1.
Overall and per-cluster patient characteristics.
| Clusters | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit A | Unit B | Unit C | Unit D | Unit E | Unit F | Unit G | Unit H | Unit I | Unit L | Unit M | Unit N | Total | P-value* | |
| Number of subjectsb | 1,145 | 1,833 | 1,012 | 934 | 330 | 690 | 591 | 1,190 | 103 | 1,388 | 721 | 434 | 10,371 | |
| Variables | Median; IQR | |||||||||||||
| Age, years | 85;80–90 | 85;80–90 | 82;77–87 | 82;75–87 | 77;70–82 | 85;80–90 | 85;80–90 | 84;79–89 | 83;76–89 | 82;76–88 | 81;76–87 | 83;77–89 | 83;78–89 | <0.001 |
| LOS, days | 7;4–11 | 7;5–10 | 7;5–11 | 9;6–13 | 27;19–36 | 18;12–25 | 15;11–20 | 9;6–12 | 17;12–24 | 7;5–11 | 8;5–11 | 9;5–14 | 8;5–14 | <0.001 |
| Barthel Index score c | 45;5–75 | 35;5–65 | 60;30–95 | 50;5–70 | 40;25–50 | 15;5–25 | 15;5–25 | 45;5–75 | 30;5–55 | 65;35–100 | 60;30–100 | 50;20–75 | 45;10–75 | <0.001 |
| Conley Scale score d | 3;2–5 | 4;2–6 | 2;1–4 | 3;1–5 | 3;2–4 | 4;3–5 | 4;2–6 | 3;1–4 | 4;2–7 | 3;1–5 | 3;1–4 | 3;2–4 | 3;2–5 | <0.001 |
| N; % | ||||||||||||||
| Falls, total | 45;13.9 | 60;18.6 | 42;13.0 | 24;7.4 | 22;6.8 | 26;8.1 | 24;7.43 | 17;5.26 | 5;1.55 | 27;8.36 | 15;4.64 | 16;4.95 | 323 | 0.025 |
| 1 time | 41;13.8 | 57;19.2 | 39;13.13 | 24;8.08 | 19;6.40 | 23;7.74 | 19;6.40 | 15;5.05 | 4;1.35 | 27;9.09 | 13;4.38 | 16;5.39 | 297 | |
| 2 times | 2;10.53 | 3;15.79 | 2;10.53 | 0 | 3;15.79 | 3;15.79 | 2;10.53 | 2;10.53 | 0 | 0 | 2;10.53 | 0 | 19 | |
| 3 times | 2;28.57 | 0 | 1;14.29 | 0 | 0 | 0 | 3;42.86 | 0 | 1;14.29 | 0 | 0 | 0 | 7 | |
| Period, n of patients | 1,145;11.0 | 1,833;17.67 | 1,012;9.76 | 934;9.01 | 330;3.18 | 690;6.65 | 591;5.70 | 1,190;11.5 | 103;0.99 | 1,388;13.4 | 721;6.95 | 434;4.18 | 10,371 | |
| 1 | 315;11.70 | 496;18.42 | 293;10.88 | 214;7.95 | 87;3.23 | 166;6.16 | 145;5.42 | 340;12.63 | 27;1.00 | 345;12.81 | 175;6.50 | 89;3.30 | 2,693 | |
| 2 | 366;12.77 | 457;15.94 | 291;10.15 | 203;7.08 | 83;2.90 | 173;6.03 | 149;5.20 | 337;11.75 | 35;1.22 | 411;14.34 | 213;7.43 | 149;5.20 | 2,867 | |
| 3 | 373;12.28 | 527;17.35 | 309;10.17 | 414;13.63 | 83;2.73 | 172;5.66 | 154;5.07 | 281;9.25 | 23;0.76 | 396;13.04 | 179;5.89 | 126;4.15 | 3,037 | |
| 4 | 91;5.13 | 353;19.90 | 119;6.71 | 103;5.81 | 77;4.34 | 179;10.1 | 142;8.00 | 232;13.08 | 18;1.01 | 236;13.3 | 154;8.68 | 70;3.95 | 1,774 | |
| Intervention, vs controls | 464;40.5 | 1,338;73.0 | 119;11.8 | 103;11.0 | 77;23.3 | 690;10 | 445;75.3 | 850;71.4 | 41;39.8 | 632;45.5 | 721;100 | 434;100 | 5,914;57.0 | |
| Sex, female | 618;53.9 | 972;53.0 | 504;49.8 | 553;59.2 | 173;52.4 | 423;61.3 | 338;57.2 | 662;55.6 | 51;51.5 | 729;52.7 | 391;54.5 | 229;53.0 | 5,643;54.5 | |
| Pharmaceuticals, yes | 985;86.3 | 1,644; 90 | 860;85 | 808;87.4 | 302;91.8 | 662;95.9 | 482;84.5 | 955;82.0 | 95;92.2 | 1,227;92 | 448;67.1 | 371;89.4 | 8,839;86.9 | |
| Orthostatic Hypotension e | ||||||||||||||
| Yes | 167;14.6 | 576;31.4 | 148;14.6 | 164;17.6 | 44;13.3 | 282;40.9 | 61;10.3 | 110;9.2 | 10;9.7 | 351;25.3 | 84;11.6 | 74;17.0 | 2,071;19.9 | |
| No | 696;60.8 | 837;45.6 | 689;68.1 | 369;39.5 | 254;76.9 | 280;40.6 | 458;77.5 | 922;77.5 | 88;85.4 | 974;70.2 | 493;68.4 | 314;72.3 | 6,374;61.5 | |
| nr | 282; 24.6 | 420;22.9 | 175;17.3 | 401;42.9 | 32;9.7 | 128;18.5 | 72;12.2 | 158;13.3 | 5;4.8 | 63;4.5 | 144;19.9 | 46;10.60 | 1,926;18.6 | |
| Patient lifts up, yes | 637;55.6 | 894;48.7 | 657;64.9 | 475;51.0 | 230;69.9 | 190;27.5 | 280;48.1 | 631;53.3 | 70;67.9 | 1,107;81.7 | 536;75.1 | 260;60.9 | 5,967;57.9 | |
aDifferences among clusters: Chi-square test for categorical variables or Kruskal–Wallis test for continuous variables
bNumber of subjects after applying inclusion criteria (patients with age ≥ 75 years old, and patients under the age of 75 but at least 18 years old, with a known risk of falling (at least 2 points in the Conley Scale)
cBarthel Index score = 0 (totally dependent) to 100 (totally independent)
dConley Scale score = 0 (no risk of falling) to 10 (high risk), scores ≥2 indicate high risk of falling
eOrthostatic hypotension—presence of orthostatic hypotension episode during a position change (no; yes—patient at risk of fall; nr: non-relevant parameter).
Care-bundle effect
The total number of falls was 323 during the overall study period (114,528 bed-days). The number of falls in the IG was 170 for 65,919 bed-days, whereas in the CG, it was 153 for 48,609 bed-days. Four patients (two in the IG and two in the CG) underwent surgery due to fall-related injuries. The patients in the IG needed minor surgery (e.g. closed shoulder reduction and repair of face skin lacerations). In contrast, control patients needed major surgery (i.e. partial hip replacement and closed reduction and internal fixation of hip fracture).
IRs of falls in both the control and intervention periods were calculated: IR of control = 3.15 for 1,000 bed-days and IR of intervention = 2.58 for 1,000 bed-days. The crude (non-adjusted) IRR of HAF for those who received the intervention with respect to those who did not was 0.82 (95% CI: 0.65–1.03), indicating a protective effect of the care-bundle, though statistical significance was not reached.
When adjusting for age, sex, and other covariates linked to patient characteristics, there was a statistically significant protective care-bundle effect: those receiving the intervention had a statistically significant decreased risk of falls compared to those who did not, with an OR of 0.71 (95%CI: 0.60–0.84; Table 2).
Table 2.
The care-bundle effect estimations in the multilevel MEGLM.
| Covariates | OR (95% CI) |
|---|---|
| Intervention, vs controls | 0.714 (0.601–0.848) |
| LOS, days | 1.044 (1.035–1.054) |
The regression was adjusted for age, sex, period, the patient’s ability to lift up, Barthel Scale, Conley Scale, orthostatic hypotension and pharmaceuticals use.
Cost-effectiveness evaluation
Table 3 shows the total undiscounted costs per cluster, period and group of exposure.
Table 3.
Total undiscounted costs (in €) per period in two groups of exposure, including intervention costs.
| Periods | Group of exposure | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposed | Control | Total per period | |||||||
| N of patients | Exposure, bed-days | Total cost | N of patients | Exposure, bed-days | Total cost | N of patients | Exposure, bed-days | Total cost | |
| Period 1 | 430 | 5,974 | 1320274.85 | 2,262 | 24,293 | 5382207.95 | 2,692 | 30,267 | 6702482.81 |
| Period 2 | 1,479 | 16,418 | 3622337.89 | 1,389 | 14,674 | 3236448.17 | 2,868 | 31,092 | 6858786.06 |
| Period 3 | 2,231 | 22,665 | 5000903.53 | 806 | 9,642 | 2125700.94 | 3,037 | 32,307 | 7126604.47 |
| Period 4 | 1,774 | 20,862 | 4605252.23 | 0 | 0 | 0 | 1,774 | 20,862 | 4605252.23 |
| Printing materials | 1689.94 | 0 | |||||||
| Aids | 48,000 | 0 | |||||||
| Distance training | 4,800 | 0 | |||||||
| Total in 4 periods | 5,914 | 65,919 | 14603258.44 | 4,457 | 48,609 | 10744357.06 | 10,371 | 114,528 | 25347615.50 |
The estimated total cost per day is 221.53 for the IG and 221.04 for the CG. The ICER obtained as incremental cost per fall prevented is €873.92. In this baseline computation, the reduction in the HAF number due to the intervention affects both the denominator through the IR and the numerator, because falls are associated with increased cost. This may lead to double-count the benefits of the intervention in terms of decreased HAF and the reduction of the associated costs. Hence, we performed a sensitivity analysis by re-computing the ICER excluding from the numerator costs related to HAF, leading to a revised ICER equal to €1644.45.
Discussion
This study evaluates the effect of a care-bundle intervention on fall prevention in hospitals. We obtained a statistically significant reduction in the risk of falls by introducing a bundle consisting of prevention strategies and staff/patient education. The care-bundle, recommended [8, 11, 14] and used in some recent literature studies [10], is advisable for both the multidisciplinary approach to a complex and multifactorial problem such as HAF and the extraordinary flexibility in suggesting descriptive and not prescriptive indications. Similarly to our study, Liu et al. [15] found a statistically significant reduction in fall rates before and after the care-bundle intervention (from 0.0434 to 0.027%, P = 0 .023). A qualitative Australian study showed the importance of patient empowerment through personal self-perception of one’s own fall risk [8]. Another recent publication reported the importance of involving patients and caregivers in falls prevention education [9]. In our study, we decided to involve both staff and patients with their families, even if we were not able to dedicate particular attention to cognitively impaired patients as suggested in another study [9].
The care-bundle included the introduction of interventions supported by the latest evidence and interventions already implemented in the units involved in the study, with the aim of a gradual change according to Evidence Based Medicine guidelines. For this reason, also the Conley scale and bed alarms have been included in the Bundle, although some recent studies [16, 17] showed that those interventions alone are not effective in reducing the incidence of falls [18].
In our study, the ICER corresponding to the incremental cost per fall prevented was €873.92. It would be of great interest to know whether the intervention can be considered cost-effective. However, no commonly accepted maximum threshold exists for an ICER expressed in these terms. To answer whether the intervention is cost-effective, we can compare the incremental cost per fall avoided with the cost of a fall. The ICER, calculated to prevent double-counting in terms of reduced number of falls and the reduction in associated costs, was equal to 1644.45. To conclude whether the intervention is cost-effective or not, this number can be compared with the average cost of a fall. Estimates of the cost of a HAF vary substantially, depending on the type of fall, the range of costs (e.g. direct vs. indirect) and the geographical area. A systematic review on the costs of falls in old age [19] reported estimates of costs per fall ranging between 944 euro estimated for Finland [20] and 10,913 USD for USA. In a meta-analysis by Alipour et al. [21], the values of ICER in the different studies ranged between 120,667 and 4280.9 dollars. We are not aware of estimates of the cost per fall for Italy. Irrespective of the geographic area of interest, estimates almost invariably consider only direct costs, thus providing a lower bound for the cost of falls from the societal perspective. The burden of these events on caregivers is well known but hard to quantify in monetary terms. Based on these considerations, we can conclude that our intervention is likely to be cost-effective.
Similar findings were reported in a 2016 multi-centre study, focusing on care homes in the UK: the incremental cost per QALY was between £4,544 and £20,889 [22]. Given that the maximum acceptability threshold for the ICER in the UK is typically set above £20,000 per QALY, the intervention can be considered cost-effective according to this estimate.
One of the strengths of our study is the high number of patients, entailing high precision in the estimation of the intervention effect. Another advantage lies in each cluster acting as its own control, with a gain in statistical power. We have adjusted for the possible confounding effects due to the transitions at various time steps from the control cluster to the exposed cluster, as required for the stepped-wedge design [23].
In this study, the statistical power could have been slightly reduced by the application of the care-bundle in three hospital units at the beginning of the trial without a previous control period.
The data were analysed later than expected due to organisational problems and the COVID-19 pandemic.
Conclusions
The care-bundle program introduced in this study led to a statistically significant decrease in the risk of falls in patients aged 75 years or older admitted to a hospital unit and it appears to be cost-effective compared to the practices routinely used.
Contributor Information
Gianfranco Di Gennaro, Department of Health Sciences, University of Catanzaro "Magna Græcia", Catanzaro, Italy.
Liliya Chamitava, Department of Diagnostics and Public Health, University of Verona, Verona, Italy.
Paolo Pertile, Department of Economics, University of Verona, Verona, Italy.
Elisa Ambrosi, Department of Diagnostics and Public Health, University of Verona, Verona, Italy.
Daniela Mosci, Hospital Hygiene and Prevention, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy.
Alice Fila, Department of Diagnostics and Public Health, University of Verona, Verona, Italy.
Mulubirhan Assefa Alemayohu, Department of Diagnostics and Public Health, University of Verona, Verona, Italy.
Lucia Cazzoletti, Department of Diagnostics and Public Health, University of Verona, Verona, Italy.
Stefano Tardivo, Department of Diagnostics and Public Health, University of Verona, Verona, Italy.
Maria Elisabetta Zanolin, Department of Diagnostics and Public Health, University of Verona, Verona, Italy.
Declaration of Conflicts of Interest
None.
Declaration of Sources of Funding
This paper was supported by a grant from the ‘Progetto Regione- Università’, Emilia-Romagna Region (Italy), ‘Research for Clinical Governance’ 2013, for the study: ‘Stepped-Wedge Cluster Randomized Controlled Trial for the Evaluation of Effectiveness of an Accidental Falls Care-Bundle Implementation in Elderly Hospital Patients’. The grant has been assigned to Liliya Chamitava. The Emilia-Romagna Region had no role in the definition of the study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication.
Registration Number
First Recruitment
28 April 2015.
References
- 1. Close JCT, Lord SR. Fall prevention in older people: past, present and future. Age Ageing 2022; 51: 1–5. 10.1093/ageing/afac105. [DOI] [PubMed] [Google Scholar]
- 2. Moreland B, Kakara R, Henry A. Trends in nonfatal falls and fall-related injuries among adults aged ≥65 years — United States, 2012–2018. MMWR Morb Mortal Wkly Rep 2020; 69: 875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haagsma JA, Olij BF, Majdan M et al. Falls in older aged adults in 22 European countries: incidence, mortality and burden of disease from 1990 to 2017. MBJ Inj Prev 26: i67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morris ME, K W. Interventions to reduce falls in hospitals: a systematic review and meta-analysis. Age Ageing 2022; 51: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tricco ACTS, Veroniki AA. Comparisons of interventions for preventing falls in older adults: a systematic review and meta-analysis. JAMA 2017; 318: 1687–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montero-Odasso M, van der Velde N, Martin FC et al. World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing 2022; 51: afac205. 10.1093/ageing/afac205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montero-Odasso M, van der Velde. World guidelines for falls prevention and management for older adults: a global initiative. Age Ageing 2022; 51: 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heng H, Slade SC, Jazayeri D et al. Patient perspectives on hospital falls prevention education. Front Public Health 2021; 9: 592440. 10.3389/fpubh.2021.592440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hazel Heng DK, Shaw L. Implementing patient falls education in hospitals: a mixed-methods trial. Healthcare 2022; 10: 1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen LLW, Li H. Development of an evidence-based care bundle protocol for preventing falls in hospitalized children: Delphi study and trial test. Nurs Open 2022; 10: 1715–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kea Turner VS. Fall prevention implementation strategies in use at 60 United States hospitals: a descriptive study. BMJ Qual Saf 2020; 29: 1000–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willem Woertman EDH, Moerbeek M, Zuidema SU, Gerritsen DL, Teerenstra S. Stepped wedge designs could reduce the required sample size in cluster randomized trials. J Clin Epidemiol 2013; 66: 752–8. [DOI] [PubMed] [Google Scholar]
- 13.Stephen Morris ND, Parkin D, Spencer A. Economic analysis in healthcare. John Wiley & Sons, Second Edition, 2012. [Google Scholar]
- 14.Louise Shaw DK. Interprofessional education to implement patient falls education in hospitals: lessons learned. Nurs Open 2023; 10: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu XZX, Song Y. Retrospective analysis and nursing management of inpatient falls case series. Medicine 2021; 100: e27977. 10.1097/MD.0000000000027977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meg E, Morris TH. Divesting from a scored hospital Fall Risk Assessment Tool (FRAT): a cluster randomized non-inferiority trial. J Am Geriatr Soc 2021; 69: 2598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Terry P, Haines MB. Disinvestment in the presence of uncertainty: description of a novel, multi-group, disinvestment trial design and protocol for an application to reduce or cease use of mobilisation alarms for preventing falls in hospitals. PLoS One 2021; 16: e0261793. 10.1371/journal.pone.0261793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jellett J, Williams C, Clayton D, Plummer V, Haines T. Falls risk score removal does not impact inpatient falls: a stepped-wedge, cluster-randomised trial. J Clin Nurs 2020; 29: 4505–13. [DOI] [PubMed] [Google Scholar]
- 19. Rapp SHK. Cost of falls in old age: a systematic review. Osteoporos Int 2010; 21: 891–902. [DOI] [PubMed] [Google Scholar]
- 20. Nurmi ILP. Incidence and costs of falls and fall injuries among elderly in institutional care. Scand J Prim Health Care 2022; 20: 118–22. [PubMed] [Google Scholar]
- 21. Alipour VA-AS, Rezapour. Cost-effectiveness of multifactorial interventions in preventing falls among elderly population: a systematic review. Bull Emerg Trauma 2021; 9: e0261793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Logan PAHJ, Gladman JRF. Multifactorial falls prevention programme compared with usual care in UK care homes for older people: multicentre cluster randomised controlled trial with economic evaluation. BMJ 2021; 7: 375. 10.1136/bmj-2021-066991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanders GDNP, Basu A. Brock recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016; 316: 1093. 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]


