Abstract
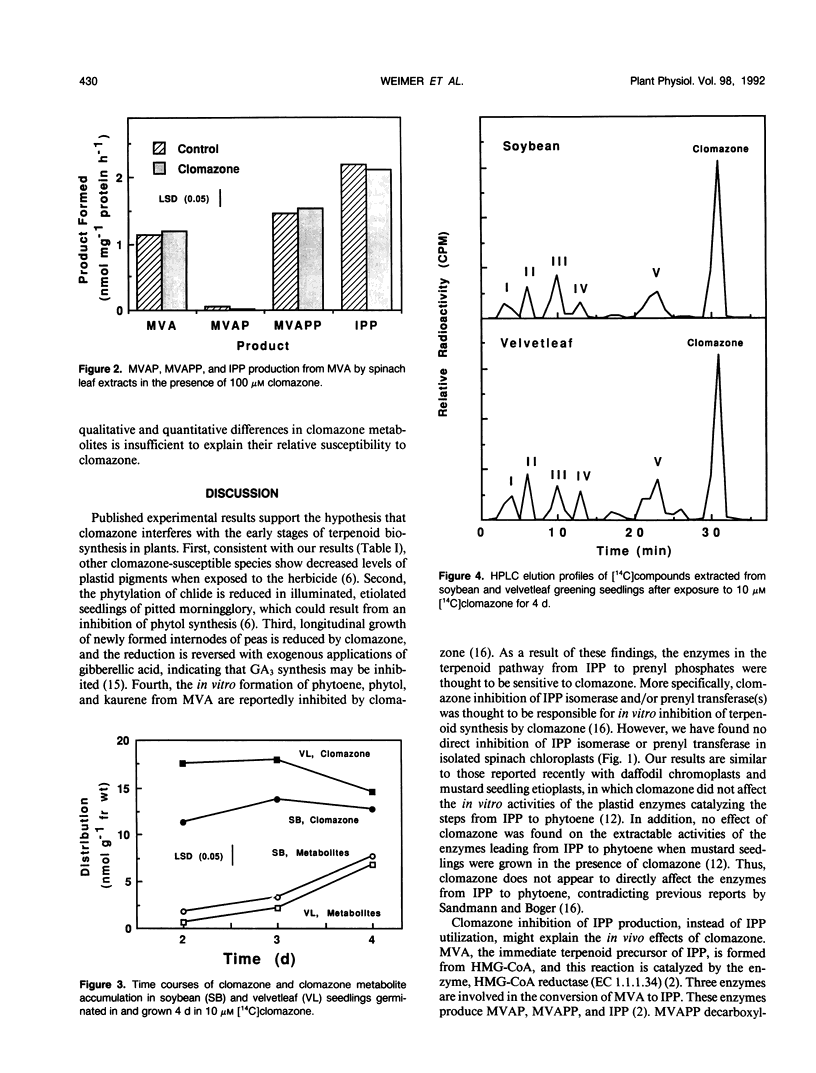
Clomazone reduced the chlorophyll and carotenoid contents of spinach (Spinacia oleracea L.), barley (Hordeum vulgare L.), velvetleaf (Abutilon theophrasti Medik.), and soybean (Glycine max L. Merr.) seedlings. The order of species sensitivity was velvetleaf > spinach > barley > soybean. Clomazone (100 micromolar) did not affect the in vitro activities of spinach isopentenyl pyrophosphate isomerase or prenyl transferase. Clomazone also did not affect the synthesis of isopentenyl pyrophosphate from mevalonic acid. Thus, clomazone had no direct in vitro effect on the synthesis of geranylgeranyl pyrophosphate from mevalonic acid. Greening seedlings of both soybean and velvetleaf metabolized clomazone. No qualitative differences in the metabolites were detected between soybean and velvetleaf. Thus, differential metabolism of clomazone to a toxic chemical that inhibits terpenoid synthesis is unlikely. Clomazone has either a mode of action not yet identified or a metabolite that is selective in that it is much more active in sensitive than tolerant species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M. A., Joyard J., Douce R. Site of synthesis of geranylgeraniol derivatives in intact spinach chloroplasts. Biochim Biophys Acta. 1980 Aug 1;631(1):210–219. doi: 10.1016/0304-4165(80)90069-0. [DOI] [PubMed] [Google Scholar]
- Dugan R. E., Rasson E., Porter J. W. Separation of water-soluble steroid and carotenoid precursors by DEAE-cellulose column chromatography. Anal Biochem. 1968 Feb;22(2):249–259. doi: 10.1016/0003-2697(68)90314-x. [DOI] [PubMed] [Google Scholar]
- Heintze A., Görlach J., Leuschner C., Hoppe P., Hagelstein P., Schulze-Siebert D., Schultz G. Plastidic Isoprenoid Synthesis during Chloroplast Development : Change from Metabolic Autonomy to a Division-of-Labor Stage. Plant Physiol. 1990 Jul;93(3):1121–1127. doi: 10.1104/pp.93.3.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalitha R., George R., Ramasarma T. Mevalonate-metabolizing enzymes in Arachis hypogaea. Mol Cell Biochem. 1989 Jun 1;87(2):161–170. doi: 10.1007/BF00219259. [DOI] [PubMed] [Google Scholar]
- Norman M. A., Liebl R. A., Widholm J. M. Uptake and metabolism of clomazone in tolerant-soybean and susceptible-cotton photomixotrophic cell suspension cultures. Plant Physiol. 1990 Mar;92(3):777–784. doi: 10.1104/pp.92.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurgeon S. L., Sathyamoorthy N., Porter J. W. Isopentenyl pyrophosphate isomerase and prenyltransferase from tomato fruit plastids. Arch Biochem Biophys. 1984 May 1;230(2):446–454. doi: 10.1016/0003-9861(84)90425-9. [DOI] [PubMed] [Google Scholar]


