Abstract
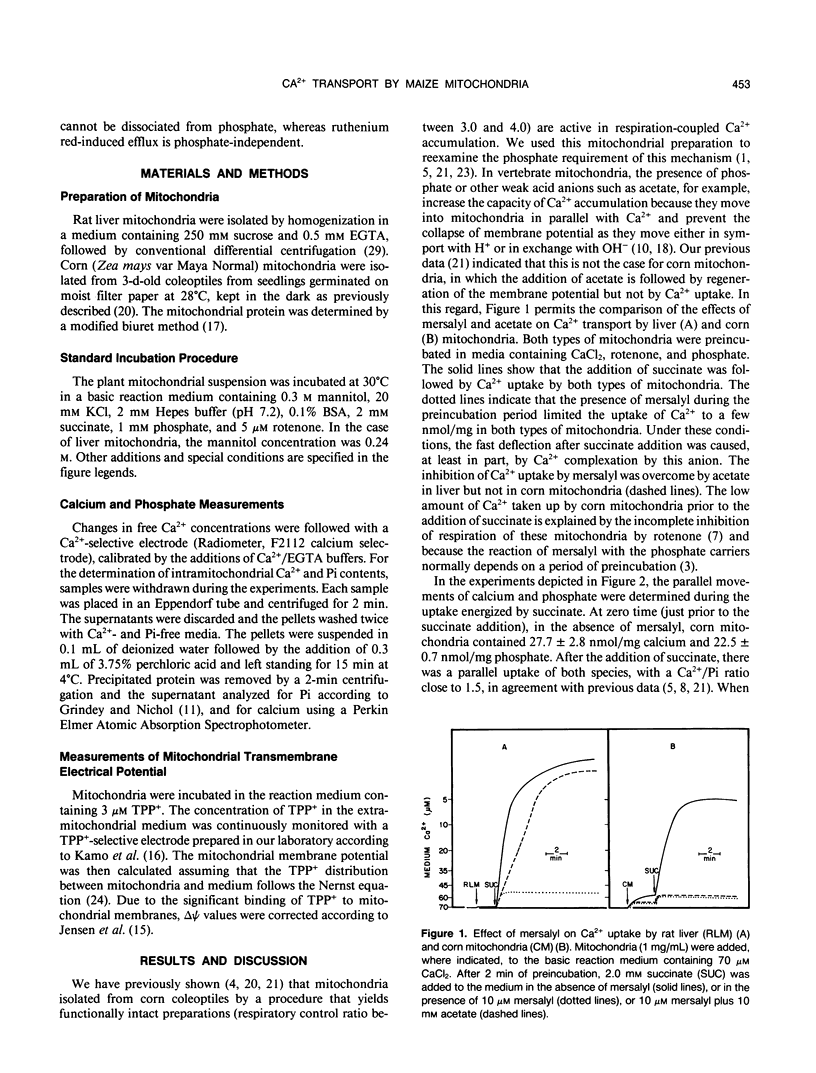
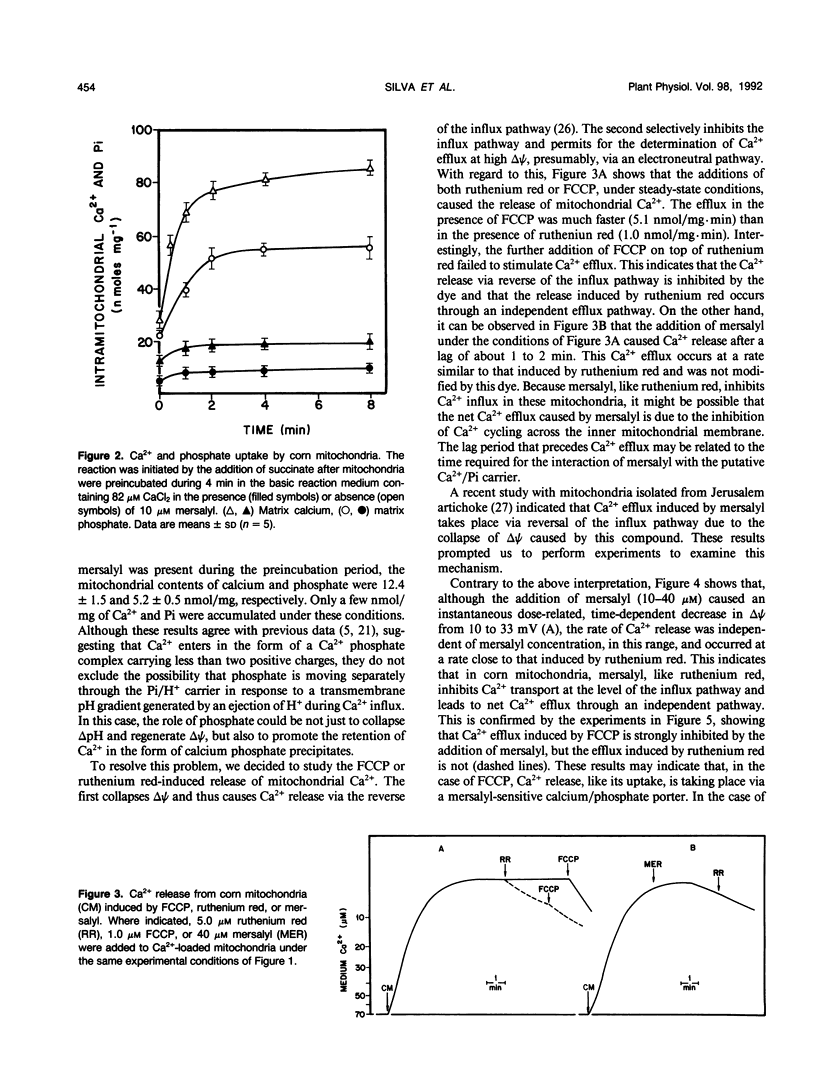
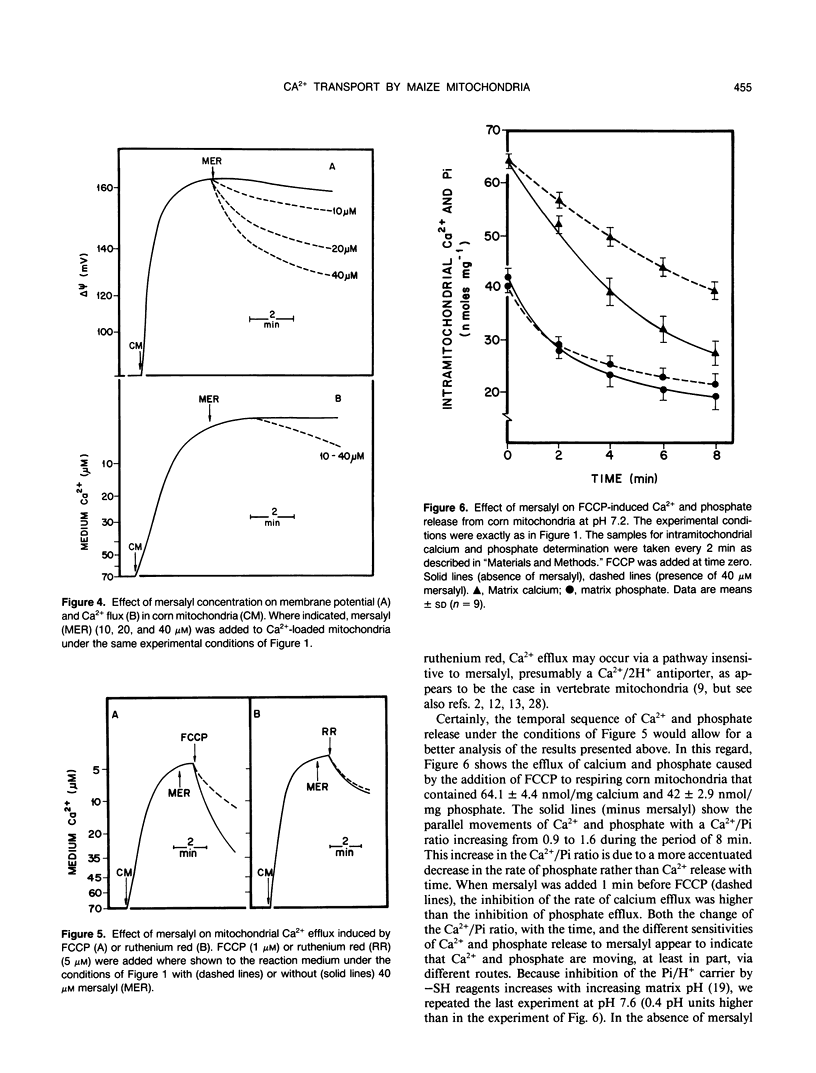
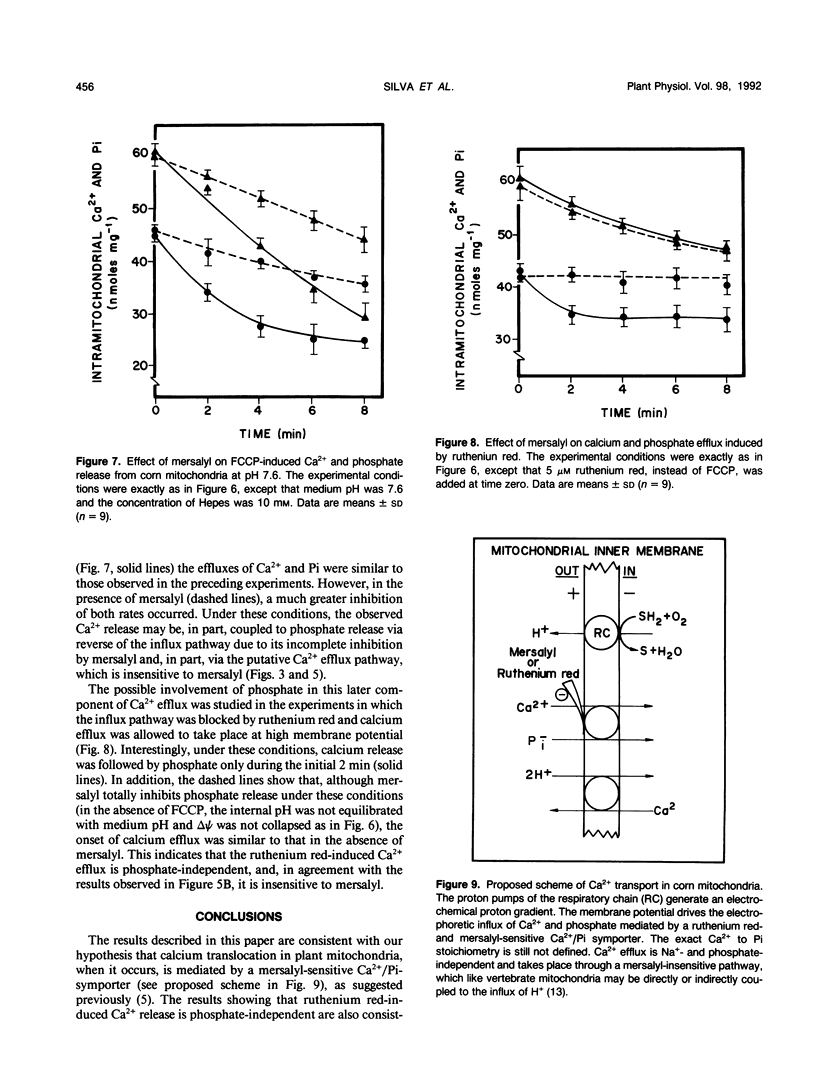
Mitochondria from some plant tissues possess the ability to take up Ca2+ by a phosphate-dependent mechanism associated with a decrease in membrane potential, H+ extrusion, and increase in the rate of respiration (AE Vercesi, L Pereira da Silva, IS Martins, CF Bernardes, EGS Carnieri, MM Fagian [1989] In G Fiskum, ed, Cell Calcium Metabolism. Plenum Press, New York, pp 103-111). The present study reexamined the nature of the phosphate requirement in this process. The main observations are: (a) Respiration-coupled Ca2+ uptake by isolated corn (Zea mays var Maya Normal) mitochondria or carbonyl cyanide p-trifluoromethoxyphenylhydrazone-induced efflux of the cation from such mitochondria are sensitive to mersalyl and cannot be dissociated from the silmultaneous movement of phosphate in the same direction. (b) Ruthenium red-induced efflux is not affected by mersalyl and can occur in the absence of phosphate movement. (c) In Ca2+-loaded corn mitochondria, mersalyl causes net Ca2+ release unrelated to a decrease in membrane potential, probably due to an inhibition of Ca2+ cycling at the level of the influx pathway. It is concluded that corn mitochondria (and probably other plant mitochondria) do possess an electrophoretic influx pathway that appears to be a mersalyl-sensitive Ca2+/inorganic phosphate-symporter and a phosphate-independent efflux pathway possibly similar to the Na2+-independent Ca2+ efflux mechanism of vertebrate mitochondria, because it is not stimulated by Na+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E., Moore A. L. Phosphate dependent, ruthenium red insensitive CA2+ uptake in mung bean mitochondria. Biochem Biophys Res Commun. 1983 Aug 12;114(3):1176–1181. doi: 10.1016/0006-291x(83)90686-1. [DOI] [PubMed] [Google Scholar]
- Antonio R. V., da Silva L. P., Vercesi A. E. Alterations in mitochondrial Ca2+ flux by the antibiotic X-537A (lasalocid-A). Biochim Biophys Acta. 1991 Feb 8;1056(3):250–258. doi: 10.1016/s0005-2728(05)80056-8. [DOI] [PubMed] [Google Scholar]
- Bryla J. Inhibitors of mitochondrial anion transport. Pharmacol Ther. 1980;10(2):351–397. doi: 10.1016/0163-7258(80)90088-1. [DOI] [PubMed] [Google Scholar]
- Carnieri E. G., Martins I. S., Vercesi A. E. The mechanism and biological role of calcium transport by plant mitochondria. Braz J Med Biol Res. 1987;20(5):635–638. [PubMed] [Google Scholar]
- Day D. A., Bertagnolli B. L., Hanson J. B. The effect of calcium on the respiratory responses of corn mitochondria. Biochim Biophys Acta. 1978 May 10;502(2):289–297. doi: 10.1016/0005-2728(78)90050-6. [DOI] [PubMed] [Google Scholar]
- Docampo R., Vercesi A. E. Ca2+ transport by coupled Trypanosoma cruzi mitochondria in situ. J Biol Chem. 1989 Jan 5;264(1):108–111. [PubMed] [Google Scholar]
- Elzam O. E., Hodges T. K. Characterization of energy-dependent ca transport in maize mitochondria. Plant Physiol. 1968 Jul;43(7):1108–1114. doi: 10.1104/pp.43.7.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiskum G., Lehninger A. L. Regulated release of Ca2+ from respiring mitochondria by Ca2+/2H+ antiport. J Biol Chem. 1979 Jul 25;254(14):6236–6239. [PubMed] [Google Scholar]
- Fonyó A. Inhibitors of mitochondrial phosphate transport. Pharmacol Ther. 1979;7(3):627–645. doi: 10.1016/0163-7258(79)90045-7. [DOI] [PubMed] [Google Scholar]
- Grindey G. B., Nichol C. A. Micro procedure for determination of pyrophosphate and orthophosphate. Anal Biochem. 1970 Jan;33(1):114–119. doi: 10.1016/0003-2697(70)90444-6. [DOI] [PubMed] [Google Scholar]
- Gunter T. E., Chace J. H., Puskin J. S., Gunter K. K. Mechanism of sodium independent calcium efflux from rat liver mitochondria. Biochemistry. 1983 Dec 20;22(26):6341–6351. doi: 10.1021/bi00295a046. [DOI] [PubMed] [Google Scholar]
- Gunter T. E., Pfeiffer D. R. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990 May;258(5 Pt 1):C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- Hansford R. G. Relation between mitochondrial calcium transport and control of energy metabolism. Rev Physiol Biochem Pharmacol. 1985;102:1–72. doi: 10.1007/BFb0034084. [DOI] [PubMed] [Google Scholar]
- Jensen B. D., Gunter K. K., Gunter T. E. The efficiencies of the component steps of oxidative phosphorylation. II. Experimental determination of the efficiencies in mitochondria and examination of the equivalence of membrane potential and pH gradient in phosphorylation. Arch Biochem Biophys. 1986 Jul;248(1):305–323. doi: 10.1016/0003-9861(86)90427-3. [DOI] [PubMed] [Google Scholar]
- Kamo N., Muratsugu M., Hongoh R., Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979 Aug;49(2):105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- Kaplan R. S., Pedersen P. L. Characterization of phosphate efflux pathways in rat liver mitochondria. Biochem J. 1983 May 15;212(2):279–288. doi: 10.1042/bj2120279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger A. L. Role of phosphate and other proton-donating anions in respiration-coupled transport of Ca2+ by mitochondria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1520–1524. doi: 10.1073/pnas.71.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligeti E., Fonyó A. Phosphate transport protein of rat heart mitochondria: location of its SH-groups and exploration of their environment. Biochim Biophys Acta. 1989 Feb 28;973(2):170–175. doi: 10.1016/s0005-2728(89)80418-9. [DOI] [PubMed] [Google Scholar]
- Martins I. S., Vercesi A. E. Some characteristics of Ca2+ transport in plant mitochondria. Biochem Biophys Res Commun. 1985 Jun 28;129(3):943–948. doi: 10.1016/0006-291x(85)91982-5. [DOI] [PubMed] [Google Scholar]
- Muratsugu M., Kamo N., Kurihara K., Kobatake Y. Selective electrode for dibenzyl dimethyl ammonium cation as indicator of the membrane potential in biological systems. Biochim Biophys Acta. 1977 Feb 4;464(3):613–619. doi: 10.1016/0005-2736(77)90035-9. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The regulation of extramitochondrial free calcium ion concentration by rat liver mitochondria. Biochem J. 1978 Nov 15;176(2):463–474. doi: 10.1042/bj1760463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls D., Akerman K. Mitochondrial calcium transport. Biochim Biophys Acta. 1982 Sep 1;683(1):57–88. doi: 10.1016/0304-4173(82)90013-1. [DOI] [PubMed] [Google Scholar]
- Saris N. E. Non-respiring rat liver mitochondria do not have a Ca2+/2H+ antiporter. Acta Chem Scand B. 1987 Feb;41(2):79–82. doi: 10.3891/acta.chem.scand.41b-0079. [DOI] [PubMed] [Google Scholar]
- Vercesi A. E., Bernardes C. F., Hoffmann M. E., Gadelha F. R., Docampo R. Digitonin permeabilization does not affect mitochondrial function and allows the determination of the mitochondrial membrane potential of Trypanosoma cruzi in situ. J Biol Chem. 1991 Aug 5;266(22):14431–14434. [PubMed] [Google Scholar]
- Vercesi A. E., Macedo D. V., Lima S. A., Gadelha F. R., Docampo R. Ca2+ transport in digitonin-permeabilized trypanosomatids. Mol Biochem Parasitol. 1990 Aug;42(1):119–124. doi: 10.1016/0166-6851(90)90119-7. [DOI] [PubMed] [Google Scholar]


