Abstract
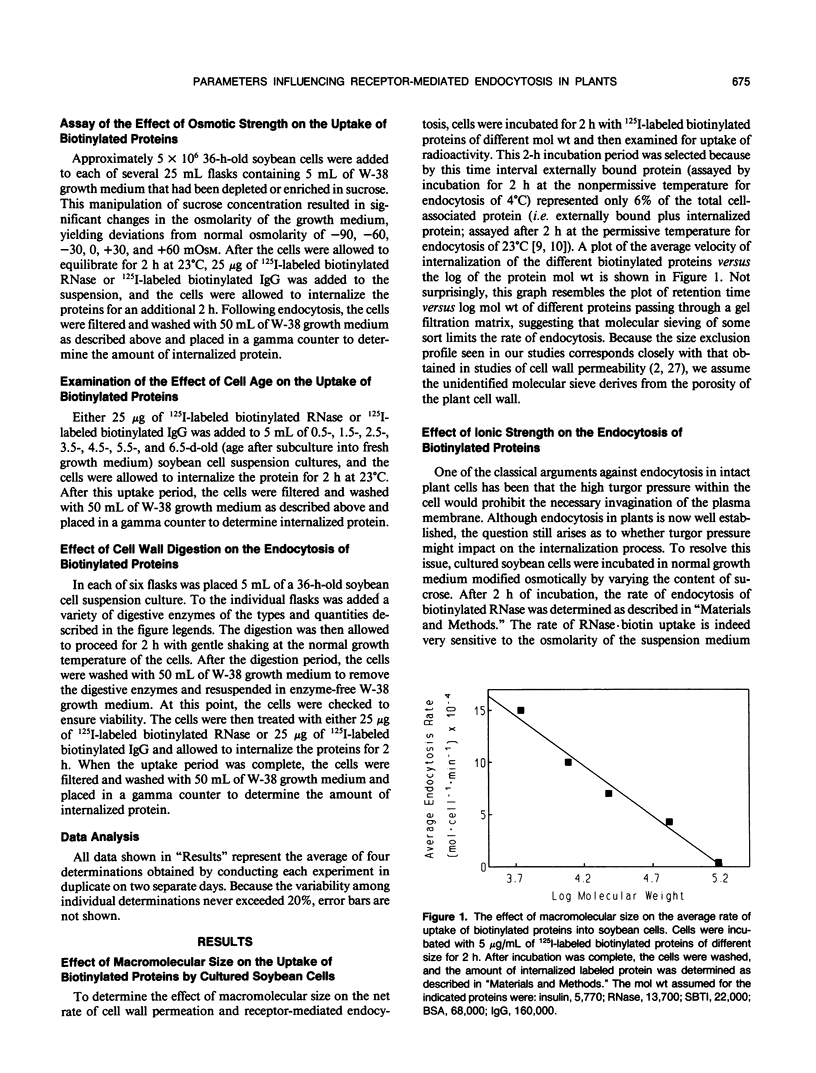
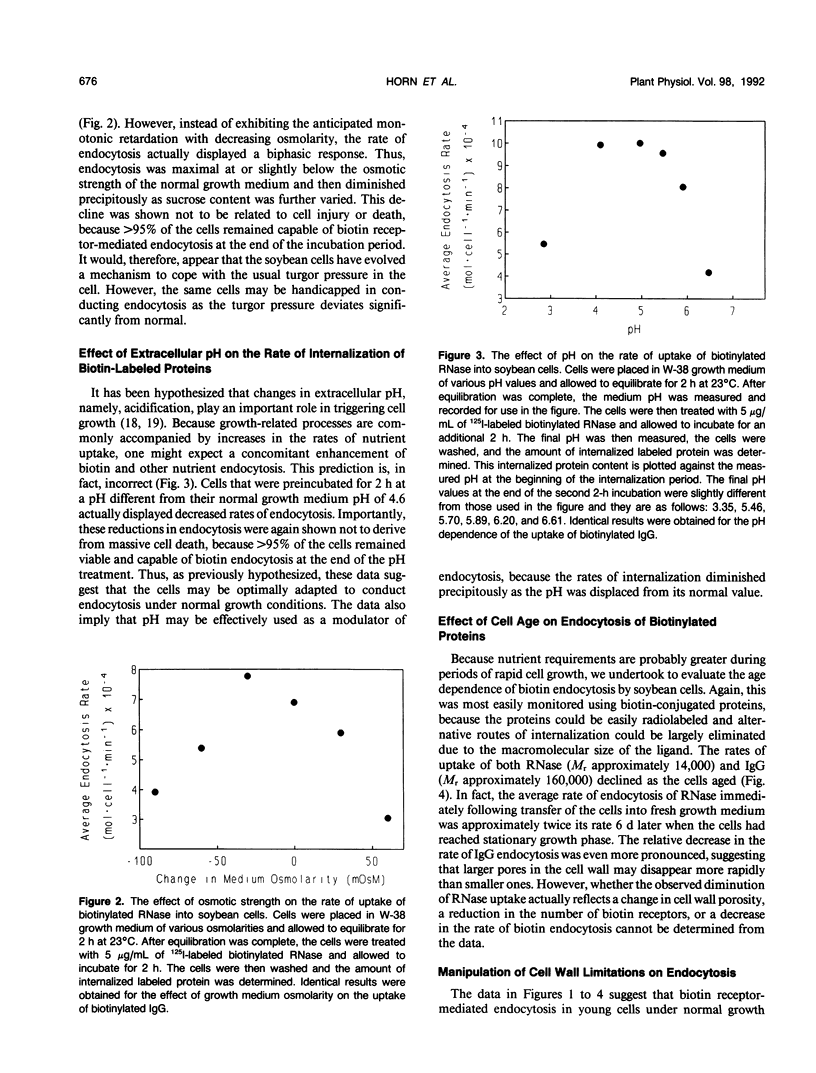
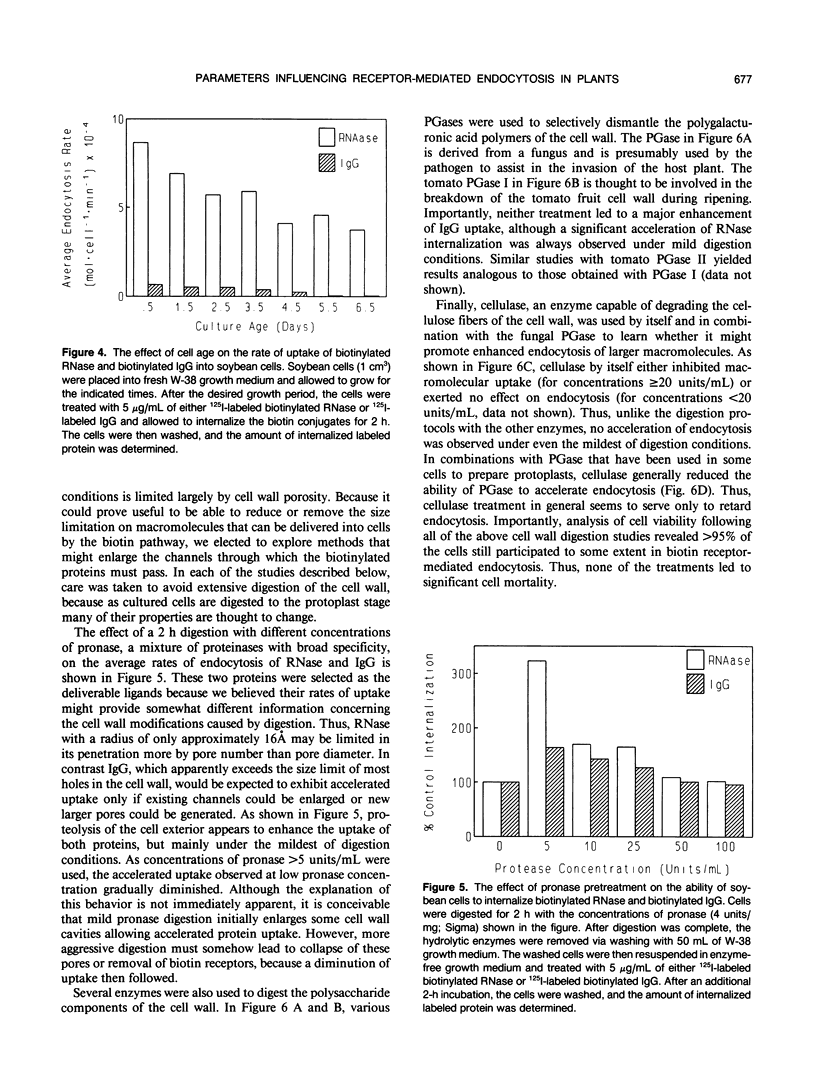
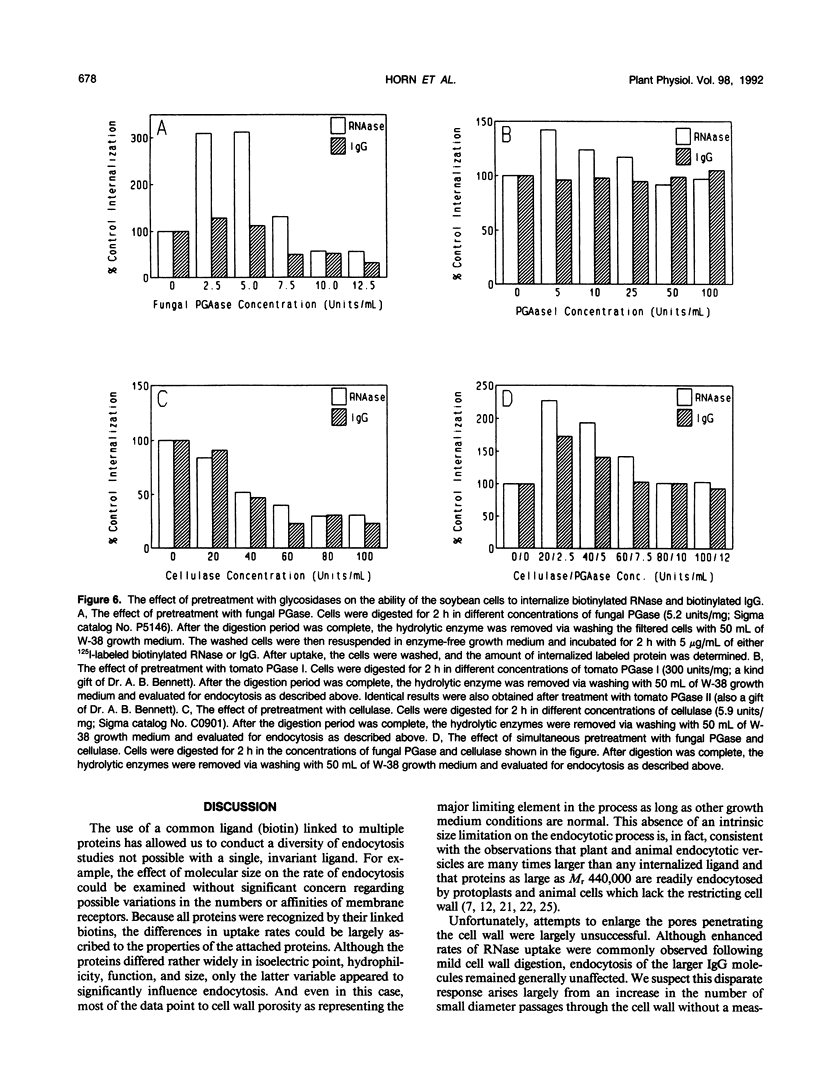
In a recent publication, we were able to demonstrate that biotin enters plant cells by receptor-mediated endocytosis and that impermeable macromolecules can be cotransported into cells by the same pathway if they are first covalently linked to biotin. In the present study, we have exploited the biotin endocytosis pathway to evaluate the variables in the cell wall and surrounding growth medium that influence the efficiency of endocytosis in plants. Under normal growth conditions, the major constraint limiting macromolecule endocytosis was found to be the size of the internalized macromolecule. Thus, a log-linear relationship with a negative slope exists between the molecular weight of the biotin-conjugated macromolecule and its rate of internalization by cultured soybean cells. This relationship, which extends from insulin (Mr approximately 5700) to immunoglobulin G (Mr approximately 160,000), is characterized by a slope of −1.04 × 105 molecules/cell/min per log Mr unit and an x intercept (no endocytosis detectable) of approximately log 160,000 daltons. Unfortunately, mild digestion with cell wall-degrading enzymes is unable to increase significantly the upper size limit of molecules that can be internalized, but uptake of lower molecular weight proteins can be enhanced by mild cell wall digestion. The optimal extracellular pH for endocytosis was found to be 4.6, i.e. near the normal pH of the cell culture medium. Furthermore, the osmotic strength at which endocytosis occurs most rapidly was observed to be isotonic to slightly hypotonic, suggesting that turgor pressure within the plant cell must not be a major determinant of endocytosis rates by cultured soybean (Glycine max) cells. Finally, cell age was found to impact significantly on the rate of macromolecule internalization, with maximal uptake rates occurring during early exponential growth and decreasing by a factor of 2 when the cells reach stationary growth phase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostol I., Low P. S., Heinstein P., Stipanovic R. D., Altman D. W. Inhibition of elicitor-induced phytoalexin formation in cotton and soybean cells by citrate. Plant Physiol. 1987 Aug;84(4):1276–1280. doi: 10.1104/pp.84.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch W. J., Mullock B. M., Luzio J. P. Rapid subcellular fractionation of the rat liver endocytic compartments involved in transcytosis of polymeric immunoglobulin A and endocytosis of asialofetuin. Biochem J. 1987 Jun 1;244(2):311–315. doi: 10.1042/bj2440311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN N. M. A SPECTROPHOTOMETRIC ASSAY FOR AVIDIN AND BIOTIN BASED ON BINDING OF DYES BY AVIDIN. Biochem J. 1965 Mar;94:23C–24C. doi: 10.1042/bj0940023c. [DOI] [PubMed] [Google Scholar]
- Horn M. A., Heinstein P. F., Low P. S. Biotin-mediated delivery of exogenous macromolecules into soybean cells. Plant Physiol. 1990 Aug;93(4):1492–1496. doi: 10.1104/pp.93.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M. A., Heinstein P. F., Low P. S. Receptor-Mediated Endocytosis in Plant Cells. Plant Cell. 1989 Oct;1(10):1003–1009. doi: 10.1105/tpc.1.10.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachim S., Robinson D. G. Endocytosis of cationic ferritin by bean leaf protoplasts. Eur J Cell Biol. 1984 May;34(1):212–216. [PubMed] [Google Scholar]
- Low P. S., Heinstein P. F. Elicitor stimulation of the defense response in cultured plant cells monitored by fluorescent dyes. Arch Biochem Biophys. 1986 Sep;249(2):472–479. doi: 10.1016/0003-9861(86)90024-x. [DOI] [PubMed] [Google Scholar]
- Malone C., Koeppe D. E., Miller R. J. Localization of lead accumulated by corn plants. Plant Physiol. 1974 Mar;53(3):388–394. doi: 10.1104/pp.53.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Enhancement of wall loosening and elongation by Acid solutions. Plant Physiol. 1970 Aug;46(2):250–253. doi: 10.1104/pp.46.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezman H. Endocytosis in yeast: several of the yeast secretory mutants are defective in endocytosis. Cell. 1985 Apr;40(4):1001–1009. doi: 10.1016/0092-8674(85)90360-5. [DOI] [PubMed] [Google Scholar]
- Saxton M. J., Breidenbach R. W. Receptor-Mediated Endocytosis in Plants is Energetically Possible. Plant Physiol. 1988 Apr;86(4):993–995. doi: 10.1104/pp.86.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepfer M., Taylor I. E. The permeability of plant cell walls as measured by gel filtration chromatography. Science. 1981 Aug 14;213(4509):761–763. doi: 10.1126/science.213.4509.761. [DOI] [PubMed] [Google Scholar]


