Figure 2. Molecular basis of human TMPRSS2 recognition by the HKU1 RBD.
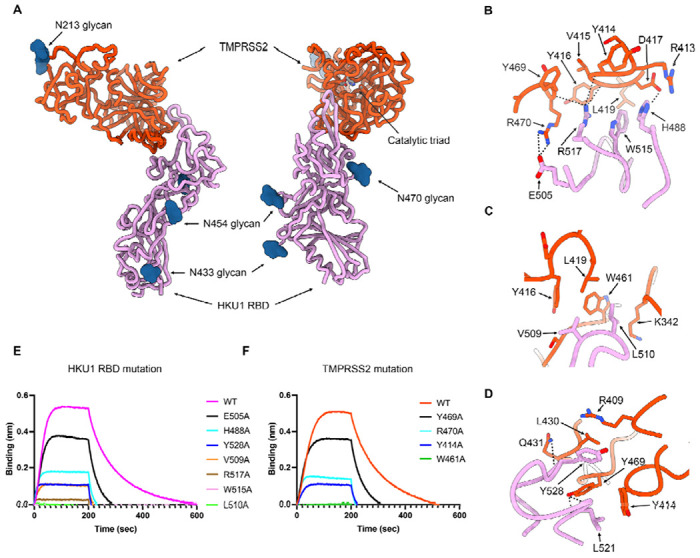
(A) Ribbon diagrams in two orthogonal orientations of the cryoEM structure of the HKU1 RBD (purple) bound to the human TMPRSS2 ectodomain (orange) at 2.9Å resolution. The TMPRSS2 catalytic triad residues (H296, D345 and S441) are shown as sticks colored by heteroatom and N-linked glycans are rendered as blue spheres. (B-D) Zoomed-in views of the interface highlighting key interactions between the HKU1 RBD and the human TMPRSS2 peptidase domain. Select electrostatic interactions are shown as black dotted lines. (E) Binding of S441A TMPRSS2 to the wildtype (WT) isolate N1 and to the H488A, E505A, V509A, L510A, W515A, R517A and Y528A HKU1 RBD interface mutants immobilized on biolayer interferometry SA biosensors. (F) Binding of the wildtype (WT) and the Y414A, W461A, Y469A and R470A human TMPRSS2 (active S441) interface mutants to the wildtype isolate N1 HKU1 RBD immobilized on biolayer interferometry SA biosensors.
