Abstract
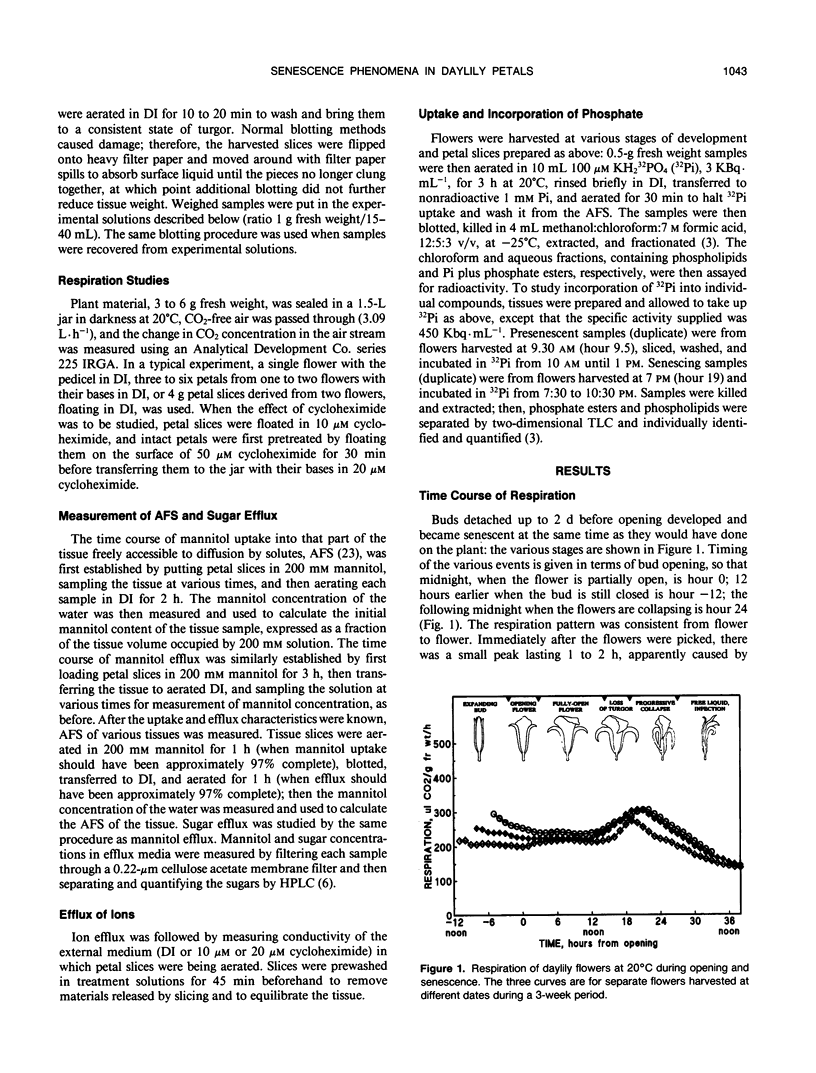
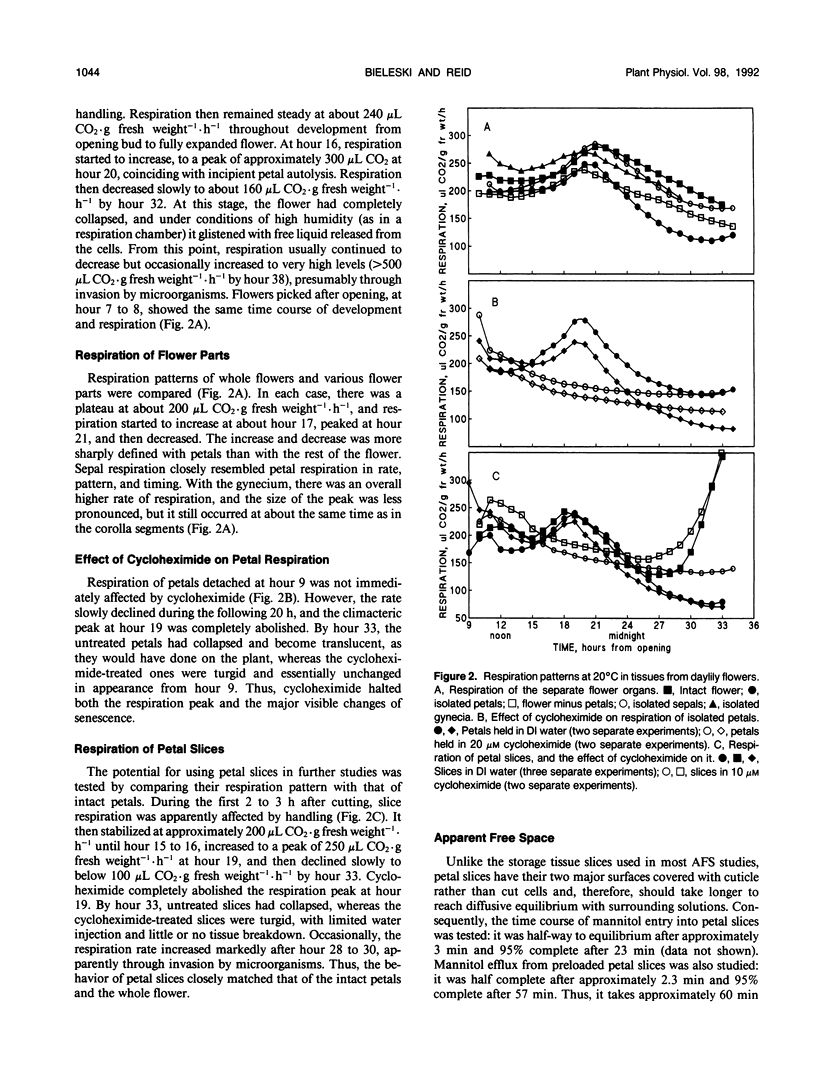
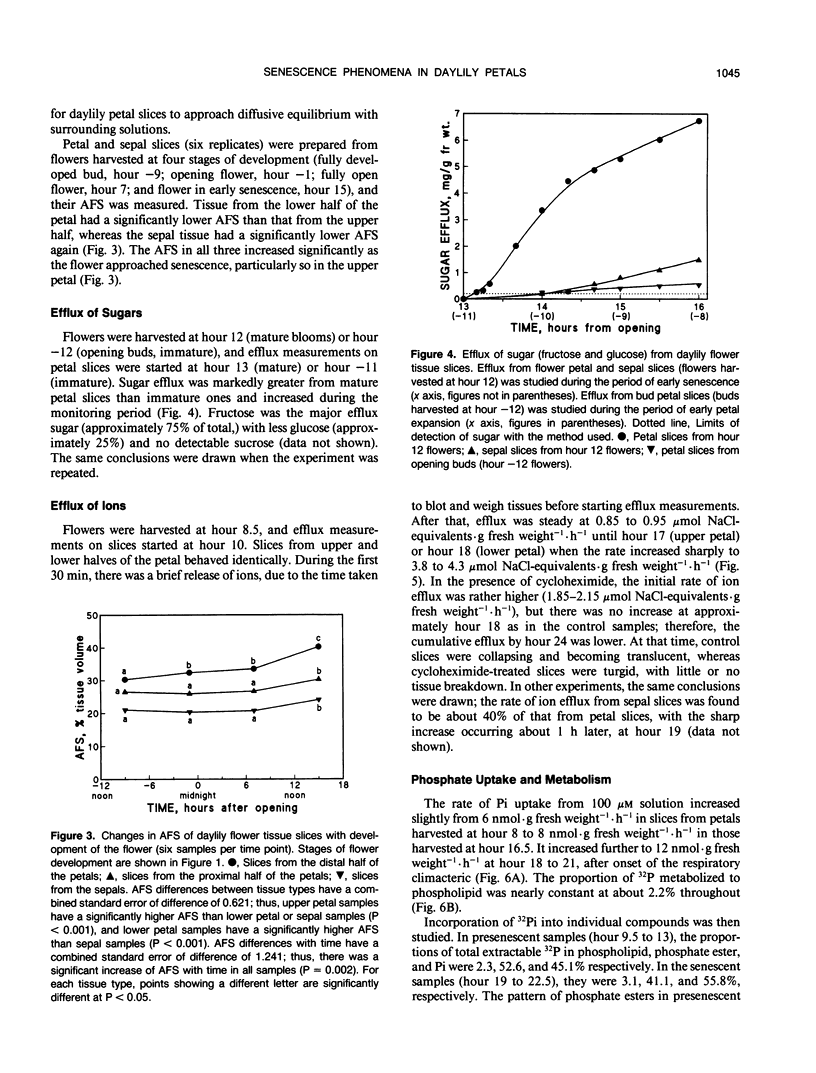
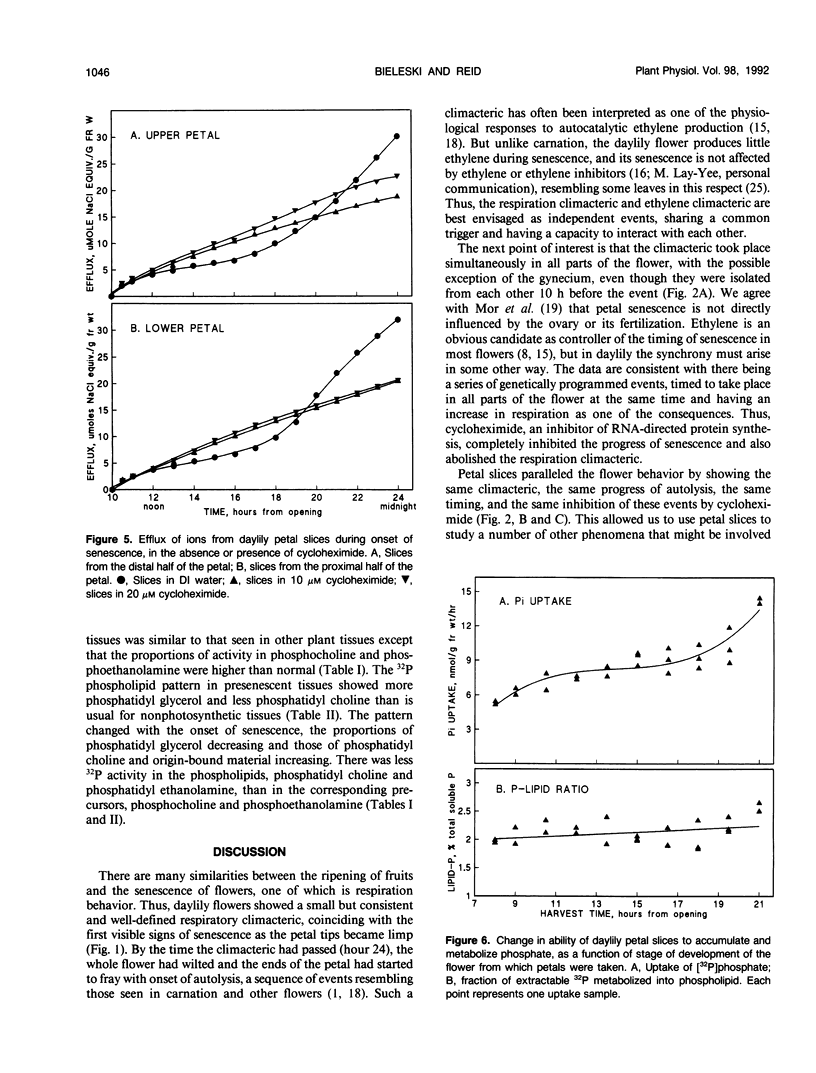
The daylily flower, Hemerocallis hybrid cv Cradle Song, develops from the opening bud to full senescence in 36 hours. Unlike other ephemeral flowers studied to date, it does not respond to ethylene, but other senescence phenomena are similar. There was a small respiration climacteric coinciding with early flower senescence, and it was also observed in isolated petals and petal slices. Cycloheximide abolished the climacteric and delayed senescence in all three systems. Petal apparent free space increased from 30% at bud opening to 38% at the onset of senescence, and sugar efflux increased from 0.2 to 2.8 milligrams per gram of fresh weight per hour during the same period. A sharp increase in ion efflux from 0.8 to 4.0 micromoles of NaCl equivalents per gram of fresh weight per hour, coinciding with the climacteric, was abolished by cycloheximide. Uptake of radiolabeled inorganic phosphate by petal slices from 100 micromolar solution increased during onset of senescence from 6 to 10 nmoles per gram of fresh weight per hour. Half was esterified; of this, 14% went into ATP, and the cellular energy charge remained high at 0.86 during senescence. The proportion incorporated into phospholipid (2.2%) did not change during senescence, but the proportion in phosphatidyl choline increased and in phosphatidyl glycerol decreased during senescence. The general phosphate ester pattern in presenescent slices closely resembled that in other plant tissues except that phospholipid precursors were more prominent (approximately 20% of total organic 32P versus 5%). In senescent slices, the proportion of hexose phosphates decreased from 40 to 15% of total organic 32P and that of phospholipid precursors increased to approximately 50%, suggesting that phospholipid synthesis was blocked early in senescence.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutelmann P., Kende H. Membrane Lipids in Senescing Flower Tissue of Ipomoea tricolor. Plant Physiol. 1977 May;59(5):888–893. doi: 10.1104/pp.59.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieleski R. L. Levels of phosphate esters in spirodela. Plant Physiol. 1968 Aug;43(8):1297–1308. doi: 10.1104/pp.43.8.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochov A., Halevy A. H., Shinitzky M. Senescence and the Fluidity of Rose Petal Membranes : RELATIONSHIP TO PHOSPHOLIPID METABOLISM. Plant Physiol. 1982 Feb;69(2):296–299. doi: 10.1104/pp.69.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faragher J. D., Wachtel E., Mayak S. Changes in the Physical State of Membrane Lipids during Senescence of Rose Petals. Plant Physiol. 1987 Apr;83(4):1037–1042. doi: 10.1104/pp.83.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Ethylene-enhanced Ion and Sucrose Efflux in Morning Glory Flower Tissue. Plant Physiol. 1975 Apr;55(4):663–669. doi: 10.1104/pp.55.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayak S., Vaadia Y., Dilley D. R. Regulation of Senescence in Carnation (Dianthus caryophyllus) by Ethylene: Mode of Action. Plant Physiol. 1977 Apr;59(4):591–593. doi: 10.1104/pp.59.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle J. C., Kende H. Ethylene Action and Loss of Membrane Integrity during Petal Senescence in Tradescantia. Plant Physiol. 1980 Jun;65(6):1067–1072. doi: 10.1104/pp.65.6.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. E., Mayak S., Shinitzky M., Halevy A. H. Acceleration of membrane senescence in cut carnation flowers by treatment with ethylene. Plant Physiol. 1982 Apr;69(4):859–863. doi: 10.1104/pp.69.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]


