Abstract
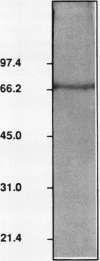
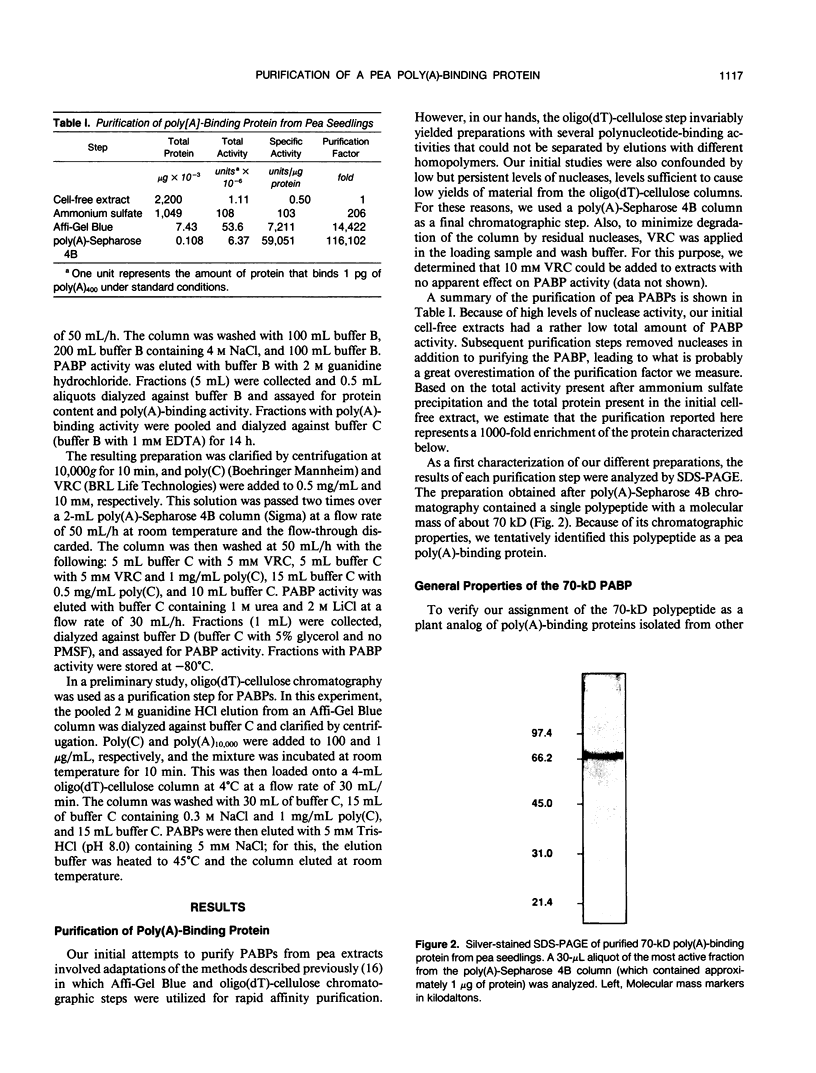
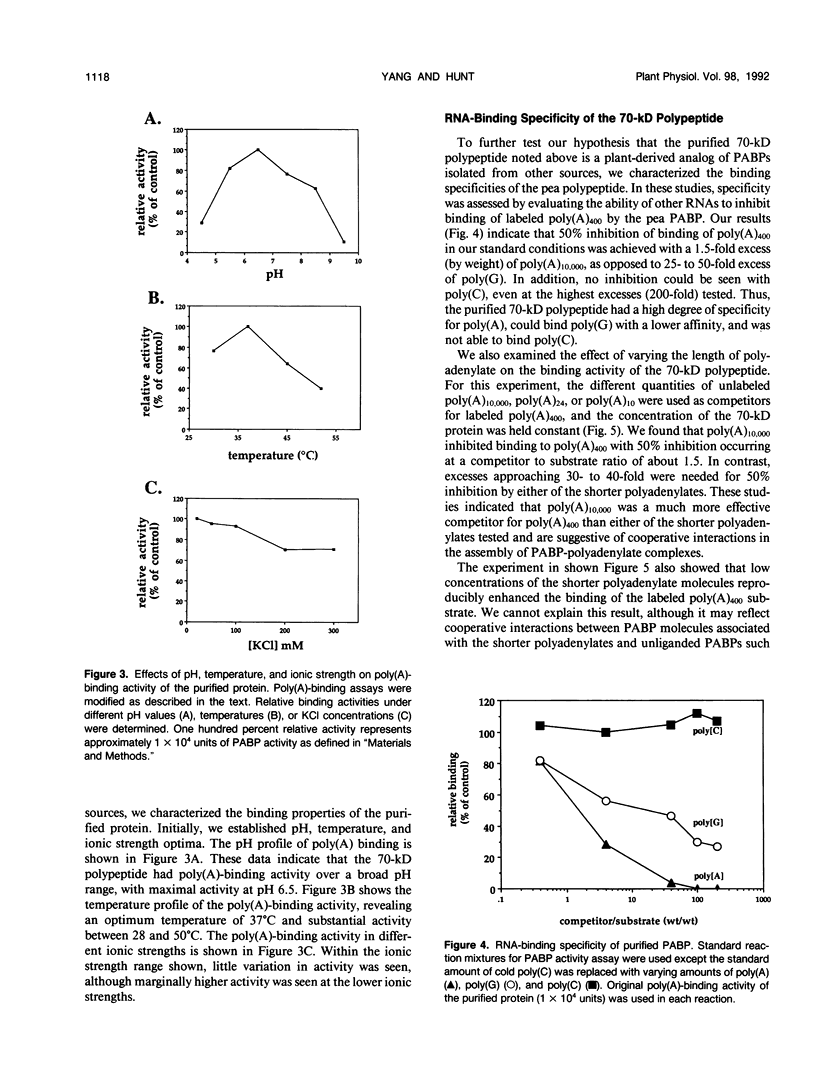
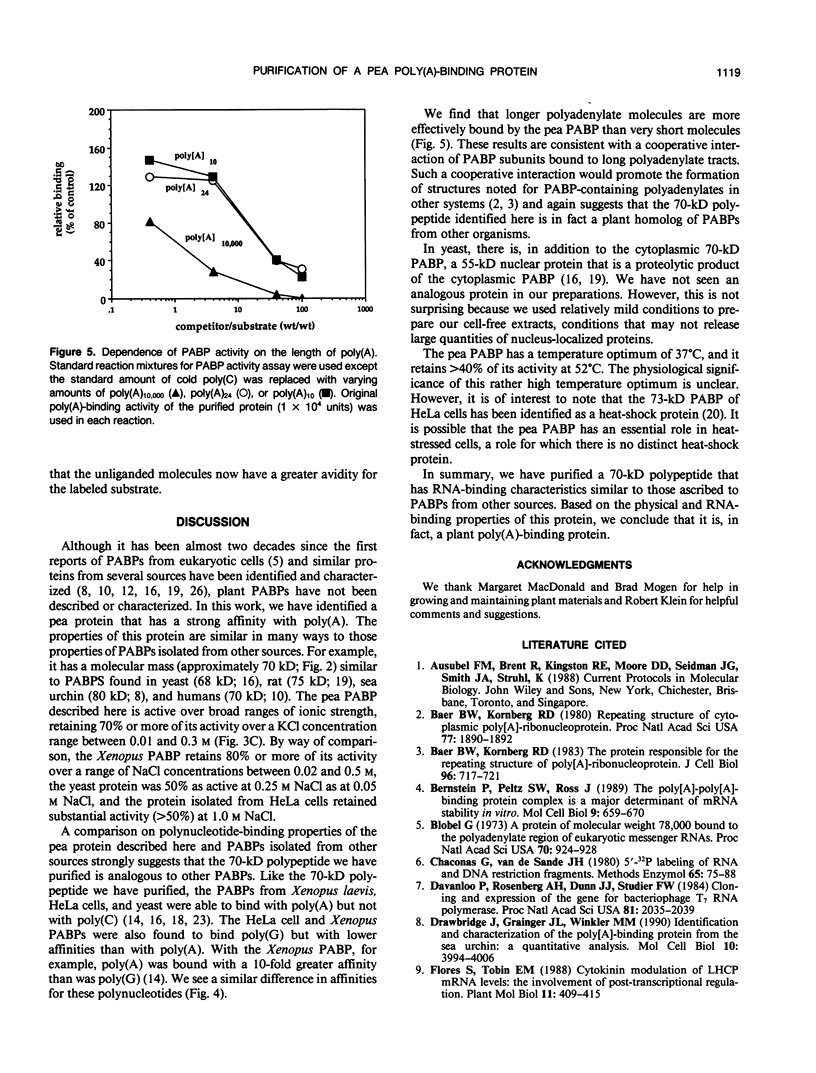
A polyadenylate-binding protein (PABP) was purified from cell-free extracts prepared from pea seedlings (Pisum sativum) by ammonium sulfate precipitation and Affi-Gel Blue and polyadenylate-Sepharose 4B affinity chromatography. The final preparation from polyadenylate-Sepharose 4B columns contained a single 70-kilodalton polypeptide with high polyadenylate-binding activity. The purified protein was active over a broad range of ionic strengths and showed temperature and pH optima of 37°C and pH 6.5, respectively. Specificity studies indicated that the pea PABP was most active with polyadenylic acids, showed some activity with polyguanylic acid, and did not bind to polycytidylic acid. Moreover, longer polyadenylate molecules were bound more effectively than shorter ones. Because these properties are similar to PABPs isolated from other sources, we conclude that we have identified, purified, and characterized a plant PABP analogous to those described in yeast and animal systems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer B. W., Kornberg R. D. Repeating structure of cytoplasmic poly(A)-ribonucleoprotein. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1890–1892. doi: 10.1073/pnas.77.4.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer B. W., Kornberg R. D. The protein responsible for the repeating structure of cytoplasmic poly(A)-ribonucleoprotein. J Cell Biol. 1983 Mar;96(3):717–721. doi: 10.1083/jcb.96.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein P., Peltz S. W., Ross J. The poly(A)-poly(A)-binding protein complex is a major determinant of mRNA stability in vitro. Mol Cell Biol. 1989 Feb;9(2):659–670. doi: 10.1128/mcb.9.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G. A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1973 Mar;70(3):924–928. doi: 10.1073/pnas.70.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaconas G., van de Sande J. H. 5'-32P labeling of RNA and DNA restriction fragments. Methods Enzymol. 1980;65(1):75–85. doi: 10.1016/s0076-6879(80)65012-5. [DOI] [PubMed] [Google Scholar]
- Davanloo P., Rosenberg A. H., Dunn J. J., Studier F. W. Cloning and expression of the gene for bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2035–2039. doi: 10.1073/pnas.81.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drawbridge J., Grainger J. L., Winkler M. M. Identification and characterization of the poly(A)-binding proteins from the sea urchin: a quantitative analysis. Mol Cell Biol. 1990 Aug;10(8):3994–4006. doi: 10.1128/mcb.10.8.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange T., de Sa C. M., Oddos J., Pictet R. Human mRNA polyadenylate binding protein: evolutionary conservation of a nucleic acid binding motif. Nucleic Acids Res. 1987 Jun 25;15(12):4771–4787. doi: 10.1093/nar/15.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lefrère V., Vincent A., Amalric F. Drosophila melanogaster poly(A)-binding protein: cDNA cloning reveals an unusually long 3'-untranslated region of the mRNA, also present in other eukaryotic species [corrected]. Gene. 1990 Dec 15;96(2):219–225. doi: 10.1016/0378-1119(90)90256-q. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Nietfeld W., Mentzel H., Pieler T. The Xenopus laevis poly(A) binding protein is composed of multiple functionally independent RNA binding domains. EMBO J. 1990 Nov;9(11):3699–3705. doi: 10.1002/j.1460-2075.1990.tb07582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochnow D., Riedel N., Agutter P. S., Fasold H. Poly(A) binding proteins located at the inner surface of resealed nuclear envelopes. J Biol Chem. 1990 Apr 25;265(12):6536–6539. [PubMed] [Google Scholar]
- Sachs A. B., Bond M. W., Kornberg R. D. A single gene from yeast for both nuclear and cytoplasmic polyadenylate-binding proteins: domain structure and expression. Cell. 1986 Jun 20;45(6):827–835. doi: 10.1016/0092-8674(86)90557-x. [DOI] [PubMed] [Google Scholar]
- Sachs A. B., Davis R. W., Kornberg R. D. A single domain of yeast poly(A)-binding protein is necessary and sufficient for RNA binding and cell viability. Mol Cell Biol. 1987 Sep;7(9):3268–3276. doi: 10.1128/mcb.7.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs A. B., Davis R. W. The poly(A) binding protein is required for poly(A) shortening and 60S ribosomal subunit-dependent translation initiation. Cell. 1989 Sep 8;58(5):857–867. doi: 10.1016/0092-8674(89)90938-0. [DOI] [PubMed] [Google Scholar]
- Sachs A. B., Kornberg R. D. Nuclear polyadenylate-binding protein. Mol Cell Biol. 1985 Aug;5(8):1993–1996. doi: 10.1128/mcb.5.8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönfelder M., Horsch A., Schmid H. P. Heat shock increases the synthesis of the poly(A)-binding protein in HeLa cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6884–6888. doi: 10.1073/pnas.82.20.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley B. W., Meagher R. B. A potential role for RNA turnover in the light regulation of plant gene expression: ribulose-1,5-bisphosphate carboxylase small subunit in soybean. Nucleic Acids Res. 1990 Jun 11;18(11):3377–3385. doi: 10.1093/nar/18.11.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skadsen R. W., Scandalios J. G. Translational control of photo-induced expression of the Cat2 catalase gene during leaf development in maize. Proc Natl Acad Sci U S A. 1987 May;84(9):2785–2789. doi: 10.1073/pnas.84.9.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Dreyfuss G. Classification and purification of proteins of heterogeneous nuclear ribonucleoprotein particles by RNA-binding specificities. Mol Cell Biol. 1988 May;8(5):2237–2241. doi: 10.1128/mcb.8.5.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. M., Meagher R. B. Transcriptional and post-transcriptional processes regulate expression of RNA encoding the small subunit of ribulose-1,5-biphosphate carboxylase differently in petunia and in soybean. Nucleic Acids Res. 1990 Jun 25;18(12):3621–3629. doi: 10.1093/nar/18.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wreschner D. H., Herzberg M. A new blotting medium for the simple isolation and identification of highly resolved messenger RNA. Nucleic Acids Res. 1984 Feb 10;12(3):1349–1359. doi: 10.1093/nar/12.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelus B. D., Giebelhaus D. H., Eib D. W., Kenner K. A., Moon R. T. Expression of the poly(A)-binding protein during development of Xenopus laevis. Mol Cell Biol. 1989 Jun;9(6):2756–2760. doi: 10.1128/mcb.9.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]