Abstract
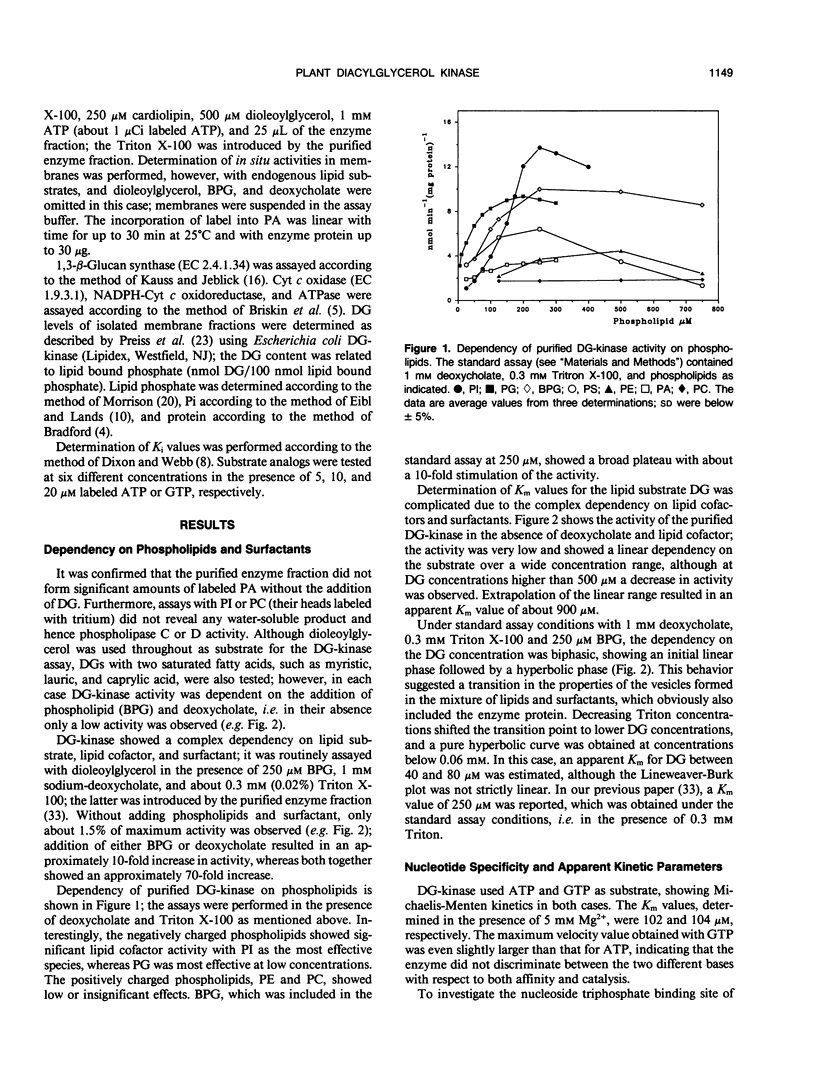
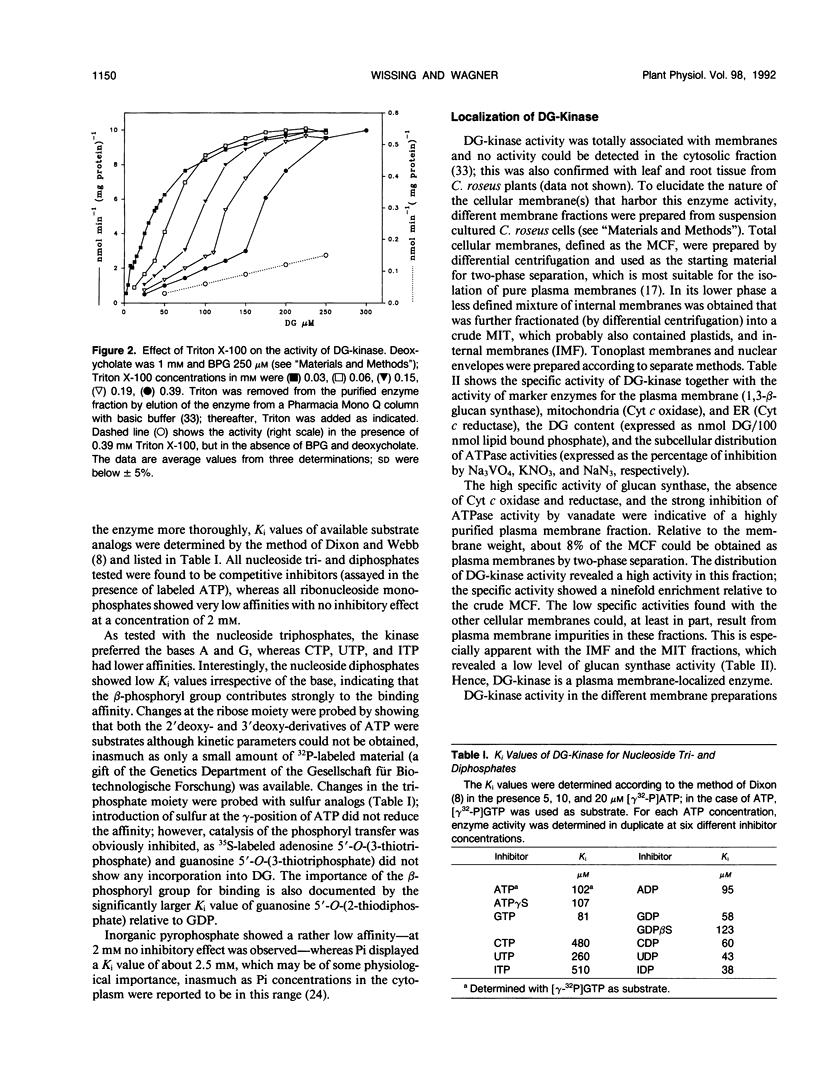
Diacylglycerol kinase (adenosine 5′-triphosphate:1,2-diacylglycerol 3-phosphotransferase, EC 2.7.1.107), purified from suspension cultured Catharanthus roseus cells (J Wissing, S Heim, KG Wagner [1989] Plant Physiol 90: 1546-1551), was further characterized and its subcellular location was investigated. The enzyme revealed a complex dependency on lipids and surfactants; its activity was stimulated by certain phospholipids, with phosphatidylinositol and phosphatidylglycerol as the most effective species, and by deoxycholate. In the presence of Triton X-100, used for its purification, a biphasic dependency upon diacylglycerol was observed and the apparent Michaelis constant values for diacylglycerol decreased with decreasing Triton concentration. The enzyme accepted both adenosine 5′-triphosphate and guanosine 5′-triphosphate as substrate and showed rather low apparent inhibition constant values for all nucleoside diphosphates tested. Diacylglycerol kinase is an intrinsic membrane protein and no activity was found in the cytosol. An investigation of different cellular membrane fractions confirmed its location in the plasma membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohnenberger E., Sandermann H., Jr Lipid dependence of diacylglycerol kinase from Escherichia coli. Eur J Biochem. 1983 May 16;132(3):645–650. doi: 10.1111/j.1432-1033.1983.tb07412.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Tsujita T., Brockman H. L. Enzymatic and physical characterization of diacylglycerol-phosphatidylcholine interactions in bilayers and monolayers. Biochemistry. 1989 Jan 10;28(1):32–40. doi: 10.1021/bi00427a006. [DOI] [PubMed] [Google Scholar]
- Das S., Rand R. P. Diacylglycerol causes major structural transitions in phospholipid bilayer membranes. Biochem Biophys Res Commun. 1984 Oct 30;124(2):491–496. doi: 10.1016/0006-291x(84)91580-8. [DOI] [PubMed] [Google Scholar]
- Eibl H., Lands W. E. A new, sensitive determination of phosphate. Anal Biochem. 1969 Jul;30(1):51–57. doi: 10.1016/0003-2697(69)90372-8. [DOI] [PubMed] [Google Scholar]
- Einspahr K. J., Thompson G. A. Transmembrane Signaling via Phosphatidylinositol 4,5-Bisphosphate Hydrolysis in Plants. Plant Physiol. 1990 Jun;93(2):361–366. doi: 10.1104/pp.93.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoh H., Kondoh H., Ono T. Diacylglycerol kinase from pig brain. Purification and phospholipid dependencies. J Biol Chem. 1983 Feb 10;258(3):1767–1774. [PubMed] [Google Scholar]
- Kanoh H., Ohno K. Partial purification and properties of diacylglycerol kinase from rat liver cytosol. Arch Biochem Biophys. 1981 Jun;209(1):266–275. doi: 10.1016/0003-9861(81)90280-0. [DOI] [PubMed] [Google Scholar]
- Kanoh H., Yamada K., Sakane F. Diacylglycerol kinase: a key modulator of signal transduction? Trends Biochem Sci. 1990 Feb;15(2):47–50. doi: 10.1016/0968-0004(90)90172-8. [DOI] [PubMed] [Google Scholar]
- Kato M., Takenawa T. Purification and characterization of membrane-bound and cytosolic forms of diacylglycerol kinase from rat brain. J Biol Chem. 1990 Jan 15;265(2):794–800. [PubMed] [Google Scholar]
- MORRISON W. R. A FAST, SIMPLE AND RELIABLE METHOD FOR THE MICRODETERMINATION OF PHOSPHORUS IN BIOLOGICAL MATERIALS. Anal Biochem. 1964 Feb;7:218–224. doi: 10.1016/0003-2697(64)90231-3. [DOI] [PubMed] [Google Scholar]
- Mazer N. A., Benedek G. B., Carey M. C. Quasielastic light-scattering studies of aqueous biliary lipid systems. Mixed micelle formation in bile salt-lecithin solutions. Biochemistry. 1980 Feb 19;19(4):601–615. doi: 10.1021/bi00545a001. [DOI] [PubMed] [Google Scholar]
- Philipp E. I., Franke W. W., Keenan T. W., Stadler J., Jarasch E. D. Characterization of nuclear membranes and endoplasmic reticulum isolated from plant tissue. J Cell Biol. 1976 Jan;68(1):11–29. doi: 10.1083/jcb.68.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Rebeille F., Bligny R., Douce R. Is the cytosolic pi concentration a limiting factor for plant cell respiration? Plant Physiol. 1984 Feb;74(2):355–359. doi: 10.1104/pp.74.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. J., Dennis E. A. Characterization of mixed micelles of phospholipids of various classes and a synthetic, homogeneous analogue of the nonionic detergent Triton X-100 containing nine oxyethylene groups. Biochim Biophys Acta. 1978 Apr 20;508(3):513–524. doi: 10.1016/0005-2736(78)90096-2. [DOI] [PubMed] [Google Scholar]
- Russ E., Kaiser U., Sandermann H., Jr Lipid-dependent membrane enzymes. Purification to homogeneity and further characterization of diacylglycerol kinase from Escherichia coli. Eur J Biochem. 1988 Jan 15;171(1-2):335–342. doi: 10.1111/j.1432-1033.1988.tb13795.x. [DOI] [PubMed] [Google Scholar]
- Sandermann H., Jr Regulation of membrane enzymes by lipids. Biochim Biophys Acta. 1978 Sep 29;515(3):209–237. doi: 10.1016/0304-4157(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Schaap D., de Widt J., van der Wal J., Vandekerckhove J., van Damme J., Gussow D., Ploegh H. L., van Blitterswijk W. J., van der Bend R. L. Purification, cDNA-cloning and expression of human diacylglycerol kinase. FEBS Lett. 1990 Nov 26;275(1-2):151–158. doi: 10.1016/0014-5793(90)81461-v. [DOI] [PubMed] [Google Scholar]
- Szkopińska A., Nowak L., Swiezewska E., Palamarczyk G. CTP-dependent lipid kinases of yeast. Arch Biochem Biophys. 1988 Oct;266(1):124–131. doi: 10.1016/0003-9861(88)90242-1. [DOI] [PubMed] [Google Scholar]
- Walsh J. P., Bell R. M. sn-1,2-Diacylglycerol kinase of Escherichia coli. Mixed micellar analysis of the phospholipid cofactor requirement and divalent cation dependence. J Biol Chem. 1986 May 15;261(14):6239–6247. [PubMed] [Google Scholar]
- Willmitzer L., Wagner K. G. The isolation of nuclei from tissue-cultured plant cells. Exp Cell Res. 1981 Sep;135(1):69–77. doi: 10.1016/0014-4827(81)90300-1. [DOI] [PubMed] [Google Scholar]
- Wissing J., Heim S., Wagner K. G. Diacylglycerol kinase from suspension cultured plant cells : purification and properties. Plant Physiol. 1989 Aug;90(4):1546–1551. doi: 10.1104/pp.90.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]


