Abstract
Background
The obesity epidemic is associated with the emergence of new kidney diseases including obesity-related glomerulopathy (ORG) and metabolic syndrome-associated disorders. However, the effects of obesity on prevalence and outcome of biopsy-proven kidney disease are not well known.
Methods
We analyzed 14,492 kidney biopsies in 18 hospitals from 1979 to 2018 in Korea. Obesity was defined as a body mass index value of ≥ 30 kg/m2.
Results
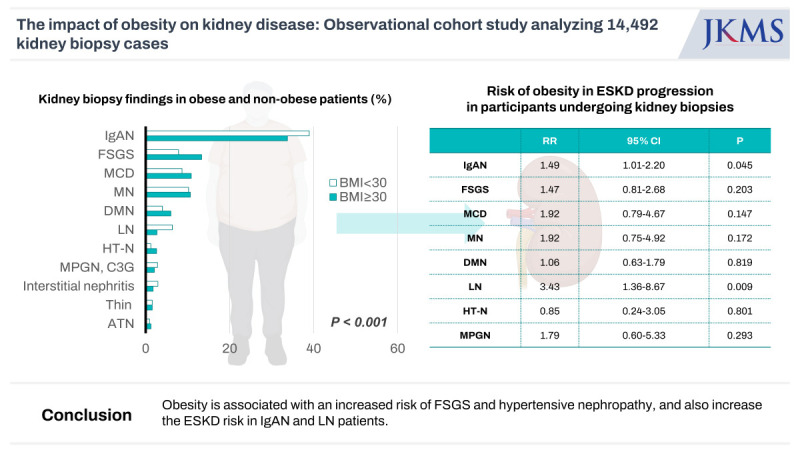
The most common disease was IgA nephropathy (IgAN) in both obese and non-obese participants (33.7% vs. 38.9%). Obesity was associated with a higher risk of focal segmental glomerulosclerosis (FSGS) and hypertensive nephropathy (HT-N) (odds ratio [OR], 1.72, 95% confidence interval [CI], 1.37–2.17; OR, 1.96, 95% CI, 1.21–3.19) and a lower risk of IgAN (OR, 0.74, 95% CI, 0.62–0.88). During the median follow up of 93.1 ± 88.7 months, obesity increased the risk of end-stage kidney disease (ESKD) in patients with IgAN (relative risk [RR], 1.49, 95% CI, 1.01–2.20) and lupus nephritis (LN) (RR, 3.43, 95% CI, 1.36–8.67). Of 947 obese individuals, ORG was detected in 298 (31.5%), and 230 participants had other kidney diseases, most commonly, IgAN (40.9%) followed by diabetic nephropathy (15.2%). Participants with ORG, when combined with other renal diseases, showed higher risks for developing ESKD compared to those with ORG alone (RR, 2.48, 95% CI, 1.09–5.64).
Conclusion
Obesity is associated with an increased risk of FSGS and HT-N, and also increase the ESKD risk in IgAN and LN patients. ORG in obese participants may have favorable renal outcomes if it occurs alone without any other renal disease.
Keywords: Chronic Kidney Disease, End Stage Kidney Disease, Glomerular Disease, Kidney Biopsy, Obesity, Obesity-Related Glomerulopathy
Graphical Abstract
INTRODUCTION
The worldwide prevalence of obesity has increased threefold over the past 40 years. The prevalence of obesity was only 3% among men and 6% among women in 1975.1 However, 39% of adults were overweight (body mass index [BMI] ≥ 25 kg/m2), and 13% were obese (BMI ≥ 30 kg/m2) globally in 2016.2 National Health and Nutritional Examination Survey 2017–March 2020 prepandemic data revealed that the age-adjusted prevalence of obesity was 41.9% and that of severe obesity (BMI ≥ 40 kg/m2) was 9.2% among adults in the United States.3
The obesity epidemic has contributed to gradual kidney damage and functional deterioration. An epidemiologic study including 630,677 participants showed that obesity was associated with a higher risk of developing a low estimated glomerular filtration rate (eGFR) and albuminuria in the general population.4 In contrast, individuals who underwent bariatric surgery had a 57% lower risk of developing end-stage kidney disease (ESKD) or doubling of serum creatinine levels over a median follow-up of 4.4 years compared to obese individuals who never underwent bariatric surgery.5 Obesity causes kidney injury by various mechanisms. First, obesity directly leads to glomerular hypertension (HTN), podocyte injury, and increase in renal inflammation.6 Second, obesity associated comorbidities, such as diabetes, HTN, kidney stones, cardiovascular disease (CVD), and medication-related acute kidney injury can also lead to kidney injury.6,7
However, the association of obesity with various immune complex-mediated glomerulonephritis (GN) is not well known. Retrospective studies demonstrated that obesity was an independent predictor of a 50% increase in serum creatinine levels8 and was associated with mesangial matrix expansion in patients with IgA nephropathy (IgAN).9 However, few studies have used kidney biopsies for information on the effects of obesity on various kidney diseases. A recent western study including 248 morbidly obese individuals reported kidney biopsy findings. Pathological characteristics of obesity-related glomerulopathy (ORG) comprise glomerulomegaly and focal segmental glomerulosclerosis (FSGS), with a specific focus on the perihilar variant.6 However, given that the spectrum of glomerular disease in Asia had features distinct from those in Western countries, the association or impact of obesity on various glomerular diseases are still unknown.10 In this study, we obtained 21,697 kidney biopsies from 1979 to 2018 in Korea and identified 969 obese participants. We investigated the effects of obesity on the prevalence and progression of glomerular diseases. We also evaluated the features and prognosis of participants with ORG alone or in combination with other renal diseases in obese participants.
METHODS
Study population
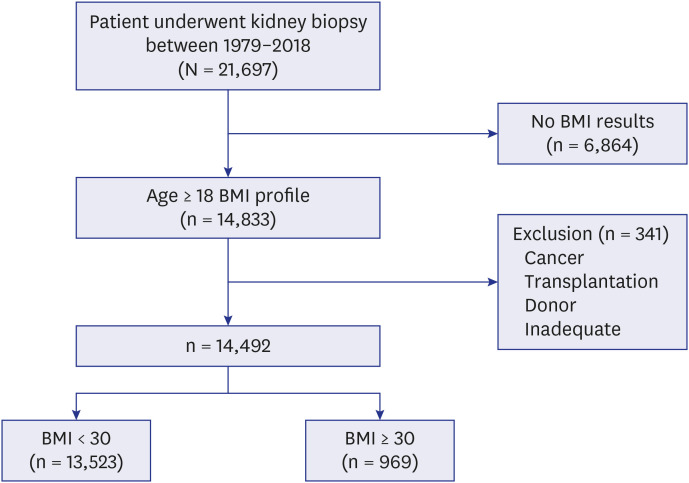
A total of 21,697 kidney biopsies were obtained from 18 hospitals from 1979 to 2018 in Korea. We first identified 14,833 participants aged older than 18 years with BMI results. Three hundred and forty-one participants with tumors such as renal cell carcinoma, transplantation, donor or inadequate samples were excluded, and 14,492 participants were finally enrolled (Fig. 1). Kidney tissue was acquired through ultrasonography-guided percutaneous gun biopsy, and the results were evaluated by a renal pathologist in each hospital. Clinical, medication, and laboratory data were acquired from the electronic medical records using the patient identification number and renal biopsy date. The data were acquired by trained research nurses.
Fig. 1. Selection of the study population.
BMI = body mass index.
Definitions
BMI was calculated using weight and height (kg/m2). Obesity was defined as a BMI of ≥ 30 kg/m2. The eGFR was calculated using the Modification of Diet in Renal Disease Study equation.11 Proteinuria was defined as a protein value of ≥ 1+ on the urine dipstick. HTN was defined as systolic blood pressure (SBP) ≥ 140 mmHg, diastolic blood pressure (DBP) ≥ 90 mmHg, or the use of antihypertensive medication. Diabetes mellitus (DM) was defined as a fasting blood glucose level of ≥ 126 mg/dL, a history of diabetes, or the use of an oral hypoglycemic agent or insulin. Treatment with immunosuppressants was defined as the use of medications within 30 days before or after a kidney biopsy. CVD was defined as myocardial infarction, angina, or stroke. ESKD and death data were collected from the registry of the Korean Society of Nephrology in April 2018, and the Statistics of Korea, and the electronic medical records.12
Kidney pathology
The evaluation of kidney pathology was described previously.13 All biopsies were evaluated by hematoxylin and eosin, Masson trichrome, periodic acid-Schiff, or periodic acid methenamine silver staining for light microscopy; immunofluorescence staining using antibodies against IgA, IgG, IgM, C3, C1q, and kappa and lambda light chains; and electron microscopy. ORG was defined as 1) glomerulomegaly alone or 2) glomerulomegaly with FSGS in obese participants.10,14 The group of ORG with other renal diseases was based on the presence of glomerulomegaly alone or in combination with FSGS out of part of the other underlying kidney disease. The pathologic diagnosis of kidney disease was made by experienced kidney pathologists.
Statistical analysis
All analyses were performed using SPSS software (version 25.0; SPSS, Chicago, IL, USA). The data are presented as the mean ± standard deviations for continuous variables and as percentages for categorical variables. Differences were analyzed using the χ2 test for categorical variables and the t-test or analysis of variance for continuous variables with post hoc Tukey’s test. For multivariate logistic regression analysis and Cox proportional hazards analysis, variables with P < 0.05 in univariate analysis, along with age and sex, were chosen. A P value of < 0.05 was considered significant. Missing data was documented as table legends.
Ethics statement
This study was approved by the Institutional Review Board (IRB) of Korea University Anam Hospital (IRB approval No. K2023-0773-003), Kyungpook National University Hospital IRB (IRB approval No. 2017-08-013), the IRB of Kyung Hee University Hospital at Gandong (IRB approval No. 2012-01-130), Kangdong Sacred Heart Hospital IRB (IRB approval No. KANGDONG 2016-06-008), IRB of Yonsei University Health System Clinical Trial Center (IRB approval No. 3-2018-0031), IRB of Korea University Guro Hospital (IRB approval No. 2017GR0082), the IRB of Eulji University Hospital (IRB approval No. EMCS 2018-06-001), the IRB of Seoul National University Boramae Medical Center (IRB approval No. 06-2011-50/106), IRB of Seoul National University Bundang Hospital (IRB approval No. B-1707/408-106), IRB, Seoul National University Hospital (IRB approval No. 1802-102-924), the IRB of Yonsei University Health System Clinical Trial Center (IRB approval No. 4-2017-0646), Pusan National University Yangsan Hospital IRB (IRB approval No. 05-2017-171), The Catholic University of Korea, Eunpyeong St. Mary’s Hospital IRB (IRB approval No. 3-116287-AB-N-01), Ewha Womans University Mokdong Hospital (IRB approval No. 2017-09-054-004), The IRB of National Health Insurance Service Ilsan Hospital Clinical Trial Center (IRB approval No. NHIMC 2017-12-017), The IRB of Chonnam National University Hospital (IRB approval No. CNUH-2018-038), Chonbuk National University Hospital IRB (IRB approval No. 2017-11-021), Hallym University Sacred Heart Hospital IRB (IRB approval No. 2017-I136). The IRB waived the requirement for obtaining informed consent due to the retrospective nature of the study.
RESULTS
Baseline characteristics
The baseline characteristics of the study population were obtained at the time of kidney biopsy (Table 1). Of the obese participants, 57% were male. Obese participants had significantly higher SBP, DBP, hemoglobin, glucose, cholesterol, and uric acid levels (P < 0.001). The prevalence of DM and HTN was higher in obese participants compared to non-obese participants, while that of cancer was significantly lower. Obese participants showed higher proteinuria and albuminuria, and the prevalence of 1+ or more dipstick proteinuria was also higher in obese participants (P < 0.05). The eGFR was not different at the time of kidney biopsy between the non-obese and obese groups. Obese participants had significantly higher C3 and C4 levels, and the prevalence of antineutrophil cytoplasmic antibody, hepatitis B surface antigen, and cryoglobulin were lower compared to non-obese participants (Table 1).
Table 1. Baseline characteristics of the participants undergoing kidney biopsies.
| Variables | BMI < 30 kg/m2 (n = 13,523) | BMI ≥ 30 kg/m2 (n = 969) | Total | P | |
|---|---|---|---|---|---|
| Age, yr | 43.2 ± 16.9 | 42.6 ± 15.8 | 43.1 ± 16.8 | 0.357 | |
| Male | 7,220 (53.4) | 553 (57.1) | 7,773 (53.6) | 0.027 | |
| BMI, kg/m2 | 23.2 ± 3.1 | 33.1 ± 3.8 | 23.9 ± 4.0 | < 0.001 | |
| SBP, mmHg | 126.1 ± 18.6 | 133.0 ± 19.8 | 126.6 ± 18.8 | < 0.001 | |
| DBP, mmHg | 78.3 ± 12.7 | 81.9 ± 12.5 | 78.6 ± 12.7 | < 0.001 | |
| DM | 1,864 (14.0) | 216 (22.7) | 2,080 (14.6) | < 0.001 | |
| HTN | 7,260 (54.4) | 715 (74.4) | 7,975 (55.8) | < 0.001 | |
| CVD | 377 (3.3) | 26 (2.9) | 403 (3.3) | 0.448 | |
| Cancer | 1,395 (10.7) | 62 (6.7) | 1,457 (10.4) | < 0.001 | |
| Hemoglobin, g/dL | 12.5 ± 2.3 | 13.4 ± 2.3 | 12.6 ± 2.4 | < 0.001 | |
| BUN, mg/dL | 21.8 ± 16.4 | 21.0 ± 14.1 | 21.7 ± 16.2 | 0.172 | |
| Creatinine, mg/dL | 1.6 ± 1.8 | 1.5 ± 1.5 | 1.6 ± 1.8 | 0.143 | |
| eGFR, (mL/min/1.73 m2 | 76.5 ± 42.1 | 76.6 ± 41.6 | 76.4 ± 42.1 | 0.935 | |
| Glucose, mg/dL | 107.8 ± 38.3 | 116.1 ± 48.5 | 108.5 ± 39.2 | < 0.001 | |
| Protein, g/dL | 6.2 ± 1.1 | 6.2 ± 1.3 | 6.2 ± 1.2 | 0.533 | |
| Albumin, g/dL | 3.4 ± 0.9 | 3.4 ± 1.0 | 3.4 ± 0.9 | 0.761 | |
| Total cholesterol, mg/dL | 217.8 ± 96.4 | 233.3 ± 104.1 | 218.2 ± 97.0 | < 0.001 | |
| Uric acid, mg/dL | 6.2 ± 2.0 | 7.0 ± 2.0 | 6.3 ± 2.0 | < 0.001 | |
| C3, mg/dL | 104.0 ± 31.2 | 125.7 ± 29.7 | 105.4 ± 31.5 | < 0.001 | |
| C4, mg/dL | 28.0 ± 13.3 | 31.1 ± 11.3 | 28.2 ± 13.2 | < 0.001 | |
| ANA positivitya | 1,752 (21.8) | 103 (18.6) | 1,855 (21.6) | 0.077 | |
| ANCA positivityb | 348 (4.7) | 12 (2.0) | 360 (4.5) | 0.002 | |
| ds DNA, IU/mLc | 34.0 ± 238.2 | 25.3 ± 304.1 | 33.4 ± 243.3 | 0.524 | |
| IgG, mg/dL | 1,087.0 ± 556.4 | 1,014.4 ± 454.3 | 1,081.5 ± 550.4 | 0.001 | |
| IgA, mg/dL | 294.7 ± 140.8 | 293.5 ± 136.9 | 294.4 ± 140.5 | 0.825 | |
| IgM, mg/dLd | 117.6 ± 111.7 | 111.3 ± 83.7 | 117.1 ± 109.8 | 0.179 | |
| HBs Ag | 560 (5.2) | 28 (3.5) | 588 (5.1) | 0.037 | |
| HCV Ab | 144 (1.6) | 10 (1.4) | 154 (1.6) | 0.615 | |
| Cryoglobuline | 297 (5.7) | 10 (2.4) | 307 (5.5) | 0.004 | |
| UA | |||||
| SG | 1.018 ± 0.009 | 1.019 ± 0.009 | 1.018 ± 0.009 | < 0.001 | |
| Protein more than 1+ | 7,676 (80.7) | 535 (86.3) | 8,211 (81.0) | < 0.001 | |
| UPCR, g/g | 3.4 ± 4.2 | 4.0 ± 4.7 | 3.4 ± 4.2 | < 0.001 | |
| UACR, g/gf | 2.1 ± 3.0 | 2.5 ± 3.0 | 2.1 ± 3.0 | 0.026 | |
Values are presented as number (%) or mean ± standard deviation.
BMI = body mass index, SBP = systolic blood pressure, DBP = diastolic blood pressure, DM = diabetes mellitus, HTN = hypertension, CVD = cardiovascular disease, BUN = blood urine nitrogen, eGFR = estimated glomerular filtration rate, C3 = complement 3, C4 = complement 4, ANA = antinuclear antibody, ANCA = antineutrophil cytoplasmic antibody, dsDNA = double-stranded DNA, HBsAg = hepatitis B surface antigen, HCV Ab = hepatitis C virus antibody, UA = urine analysis, SG = specific gravity, UPCR = urine protein creatinine ratio, UACR = urine albumin creatinine ratio.
aANA was measured in 8,579 participants; bANCA was measured in 7,955 participants; cdsDNA was measured in 4,976 participants; dIgM was measured in 7,734 participants; eCryoglobulin was measured in 5,608 participants; fUACR was measured in 4,422 participants.
Prevalence of obesity in participants undergoing kidney biopsies
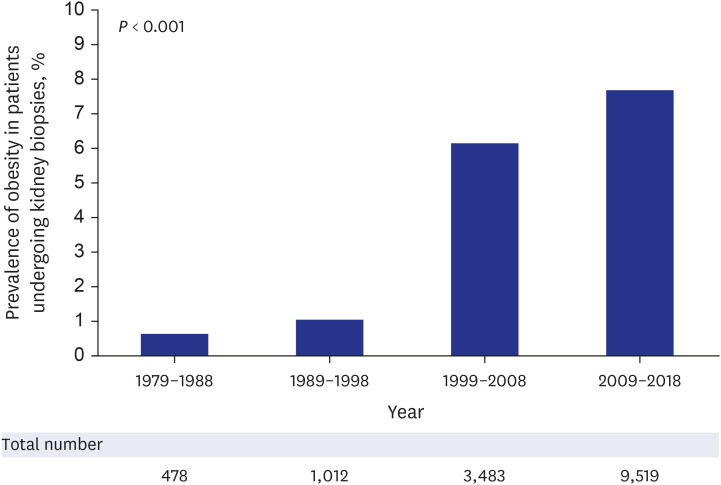
The prevalence of obesity is continuously increasing among participants undergoing kidney biopsies. The prevalence of obesity increased by 12.8-fold in 2009–2018 compared to 1979–1988 (7.7% vs. 0.6%) (Fig. 2). The prevalence of overweight status also increased from 12.3% in 1979–1988 to 36.4% in 2009–2018.
Fig. 2. Prevalence of obesity in participants undergoing kidney biopsies from 1979–1988 to 2009–2018.
Association of obesity with biopsy-proven kidney disease
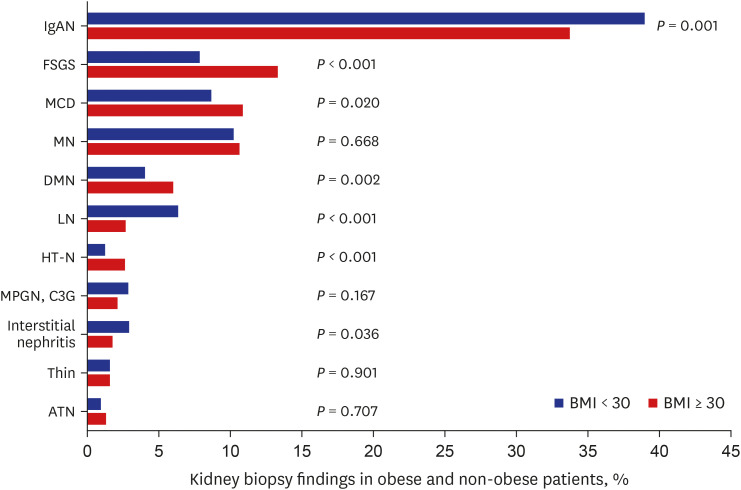
The biopsy findings differed significantly according to the obesity status (P < 0.001). The most common kidney disease in both obese and non-obese participants was IgAN (33.7% and 38.9%, respectively). In participants with obesity, FSGS was the second most common GN (13.3%), followed by minimal change disease (MCD) (10.8%), membranous nephropathy (MN) (10.6%), diabetic nephropathy (DMN) (6.0%), lupus nephritis (LN) (2.7%), and hypertensive nephropathy (HT-N) (2.6%). MN (10.2%), MCD (8.7%), FSGS (7.8%), LN (6.3%), DMN (4.0%), and interstitial nephritis (2.9%) were the most common in participants without obesity (Fig. 3).
Fig. 3. Prevalence of kidney disease confirmed by pathology according to obesity.
BMI = body mass index, IgAN = IgA nephropathy, FSGS = focal segmental glomerulosclerosis, MCD = minimal change disease, MN = membranous nephropathy, DMN = diabetic nephropathy, LN = lupus nephritis, HT-N = hypertensive nephropathy, MPGN = membranoproliferative glomerulonephritis, C3G = complement 3 glomerulopathy, ATN = acute tubular necrosis.
In unadjusted analysis, obesity was positively related to FSGS, MCD, DMN, and HT-N (P ≤ 0.021). After adjustments, FSGS and HT-N showed significant associations (odds ratio [OR], 1.72, 95% confidence interval [CI], 1.37–2.17; OR, 1.96, 95% CI, 1.21–3.19). IgAN, LN, and interstitial nephritis were negatively associated with obesity in unadjusted analysis (P ≤ 0.038), and only IgAN was found to be significantly associated with obesity (OR, 0.74, 95% CI, 0.62–0.88) after adjustment (Table 2).
Table 2. Association between obesitya and biopsy-proven kidney disease.
| Kidney disease | Unadjusted | Adjustedb | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| IgAN | 0.80 | 0.70–0.92 | 0.001 | 0.74 | 0.62–0.88 | 0.001 |
| FSGS | 1.81 | 1.49–2.20 | < 0.001 | 1.72 | 1.37–2.17 | < 0.001 |
| MCD | 1.28 | 1.04–1.59 | 0.021 | 0.89 | 0.67–1.19 | 0.438 |
| MN | 1.05 | 0.85–1.29 | 0.668 | 1.05 | 0.79–1.38 | 0.756 |
| DMN | 1.53 | 1.16–2.03 | 0.003 | 1.41 | 0.99–2.02 | 0.060 |
| LN | 0.41 | 0.27–0.61 | < 0.001 | 0.61 | 0.36–1.05 | 0.072 |
| HT-N | 2.16 | 1.41–3.30 | < 0.001 | 1.96 | 1.21–3.19 | 0.007 |
| MPGN | 0.73 | 0.46–1.15 | 0.169 | 0.98 | 0.58–1.67 | 0.946 |
| TIN | 0.60 | 0.37–0.97 | 0.038 | 0.71 | 0.39–1.29 | 0.262 |
| ATN | 1.36 | 0.75–2.46 | 0.318 | 1.20 | 0.57–2.49 | 0.634 |
OR = odds ratio, CI = confidence interval, IgAN = IgA nephropathy, FSGS = focal segmental glomerulosclerosis, MCD = minimal change disease, MN = membranous nephropathy, DMN = diabetic nephropathy, LN = lupus nephropathy, HT-N = hypertensive nephropathy, MPGN = membranoproliferative glomerulonephritis, TIN = tubulointerstitial nephritis, ATN = acute tubular necrosis.
aObesity was defined as body mass index ≥ 30 kg/m2.
bThe risks of kidney disease were adjusted by age, sex, systolic blood pressure, serum albumin, glucose, estimated glomerular filtration rate, hemoglobin, total cholesterol, cancer, and cardiovascular disease.
Effects of obesity on ESKD progression and mortality in various kidney diseases
We assessed the risk of developing ESKD in participants undergoing kidney biopsies according to the type of kidney diseases. ESKD was developed in 1,842 patients during 93.1 ± 88.7 months of mean follow-up. The risks of ESKD in obese participants were elevated in those with MCD and LN in unadjusted analysis (P ≤ 0.037). In multivariate analysis, obesity demonstrated a 1.49-fold increase in ESKD development in patients with IgAN (95% CI, 1.01–2.20) and a 3.43-fold increase in LN patients (95% CI, 1.36–8.67) (Table 3).
Table 3. Risk of obesitya in end-stage kidney disease progression in participants undergoing kidney biopsies.
| Kidney disease | Unadjusted | Adjustedb | ||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P | RR | 95% CI | P | |
| IgAN | 1.29 | 0.92–1.79 | 0.140 | 1.49 | 1.01–2.20 | 0.045 |
| FSGS | 0.91 | 0.56–1.51 | 0.254 | 1.47 | 0.81–2.68 | 0.203 |
| MCD | 2.25 | 1.06–4.78 | 0.035 | 1.92 | 0.79–4.67 | 0.147 |
| MN | 1.81 | 0.81–3.74 | 0.112 | 1.92 | 0.75–4.92 | 0.172 |
| DMN | 0.82 | 0.51–1.31 | 0.411 | 1.06 | 0.63–1.79 | 0.819 |
| LN | 2.61 | 1.06–6.43 | 0.037 | 3.43 | 1.36–8.67 | 0.009 |
| HT-N | 0.89 | 0.31–2.54 | 0.829 | 0.85 | 0.24–3.05 | 0.801 |
| MPGN | 1.88 | 0.82–4.32 | 0.134 | 1.79 | 0.60–5.33 | 0.293 |
RR = relative risk, CI = confidence interval, IgAN = IgA nephropathy, FSGS = focal segmental glomerulosclerosis, MCD = minimal change disease, MN = membranous nephropathy, DMN = diabetic nephropathy, LN = lupus nephropathy, HT-N = hypertensive nephropathy, MPGN = membranoproliferative glomerulonephritis.
aObesity was defined as body mass index ≥ 30 kg/m2.
bThe risks of kidney disease were adjusted by age, sex, systolic blood pressure, serum albumin, glucose, estimated glomerular filtration rate, hemoglobin, total cholesterol, cancer, and cardiovascular disease.
In contrast, obesity was not associated with higher mortality in any type of kidney diseases (Supplementary Table 1).
Renal biopsy findings in obese participants
Of 947 obese participants, ORG was detected only in 298 (31.5%) participants and 649 (68.5%) participants showed no evidence of ORG. In addition, 77.8% of participants with ORG (230 out of 298) were found to have combined other glomerular diseases such as IgAN (40.9%), followed by DMN (15.2%), MN (13.5%), MCD (8.3%), and LN (3.9%). For further analysis, we divided obese participants into 3 groups; Group 1 is only ORG, Group 2 is ORG + other glomerular diseases and Group 3 is no ORG. The most common type of glomerular diseases in Group 3 was IgAN (34.4%), followed by MCD (13.3%), FSGS (11.2%), MN (10.8), DMN (3.4), HT-N (2.6), and LN (2.3%).
Clinical manifestations of ORG
The participants in Group 2 were significantly older (P = 0.022). The proportion of males was 63.2%, 49.6%, and 59.3% in Groups 1, 2, and 3, respectively (P = 0.021). A history of DM was more prevalent in both Groups 1 and 2 than in Group 3 (30.9% vs. 30.9% vs. 18.7%, P < 0.001). Immunosuppressants, such as cyclosporine and mycophenolate mofetil, were most frequently administered to participants in Group 3 (P ≤ 0.005). Baseline eGFRs, hemoglobin, and serum albumin levels were significantly lower in Group 2 than in Groups 1 or 3 (P ≤ 0.020). IgA levels were significantly lower in Group 1 compared to Groups 2 and 3 (Supplementary Table 2). We performed a subgroup analysis of baseline eGFRs in each renal disease. The baseline eGFR of participants with DMN, IgAN, and LN was significantly lower in Group 2 than in Group 3 (P < 0.05). Multivariate logistic analysis revealed that higher serum albumin levels, lower IgA levels, and a history of DM were significantly related to ORG without other renal diseases (Group 1) (Supplementary Table 3).
Renal outcomes for participants with ORG
During 93.1 ± 88.7 months of follow-up, 7 patients (10.3%) in Group 1 progressed to ESKD, while 20.9% in Group 2 and 11.1% in Group 3 progressed, showing the significantly different risk according to groups. Group 2 showed a 2.48-fold risk of developing ESKD compared to Group 1 in multivariate analysis (RR, 2.48, 95% CI, 1.09–5.64). Similarly, the risk of a 30% decline in the eGFR was also significantly higher in Group 2 than in Group 1 (RR, 1.90, 95% CI, 1.03–3.51) (Table 4). Group 2 had a significantly increased risk of ESKD (RR, 1.52, 95% CI, 1.02–2.27) compared to Group 3 in multivariate analysis. Neither the risk of ESKD nor the 30% decline in the eGFR was significantly different between Groups 1 and 3.
Table 4. Risks of renal outcomes in participants with obesity-related glomerulopathy.
| Variables | Unadjusted | Age, sex adjusted | Multivariatea | ||||
|---|---|---|---|---|---|---|---|
| RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | ||
| ESKD | |||||||
| ORG (Group 1) | 0.018 | 0.021 | 0.044 | ||||
| ORG with other renal disease (Group 2) | 2.34 (1.05–5.20) | 0.037 | 2.35 (1.06–5.25) | 0.037 | 2.48 (1.09–5.64) | 0.030 | |
| Other renal disease (Group 3) | 1.45 (0.66–3.18) | 0.351 | 1.47 (0.67–3.23) | 0.339 | 1.71 (0.76–3.80) | 0.192 | |
| 30 % decline in eGFR | |||||||
| ORG (Group 1) | 0.149 | 0.104 | 0.122 | ||||
| ORG with other renal disease (Group 2) | 1.70 (0.97–2.97) | 0.063 | 1.94 (1.05–3.57) | 0.034 | 1.90 (1.03–3.51) | 0.041 | |
| Other renal disease (Group 3) | 1.42 (0.83–2.44) | 0.203 | 1.71 (0.94–3.10) | 0.080 | 1.76 (0.97–3.21) | 0.063 | |
RR = relative risk, CI = confidence interval, ESKD = end-stage kidney disease, ORG = obesity-related glomerulopathy, eGFR = estimated glomerular filtration rate.
aMultivariate analysis was adjusted by age, sex, eGFR, body mass index, serum albumin, hypertension and diabetes mellitus.
DISCUSSION
In this study, we have demonstrated the followings. First, the most common kidney biopsy finding in obese participants who underwent a kidney biopsy in Korea was IgAN (33.7%) although the prevalence of IgAN was significantly lower in obese participants than in non-obese participants. Second, although obesity independently increased the risk for FSGS and HT-N, it did not increase the risk of ESKD in these participants. However, obesity significantly increased the risk of ESKD in patients with IgAN and LN during the 93.1 months of follow-up. Third, 31 percent of the obese participants undergoing kidney biopsy had ORG. Among them, only 22.8% of the participants had ORG alone while 77.2% had combined other glomerular diseases. Higher serum albumin levels, lower IgA levels, and a history of DM were significantly related to ORG without other renal diseases. ORG participants without other renal diseases showed more favorable renal outcomes. The risks of ESKD and renal function decline were significantly increased in participants with ORG accompanied by another renal diseases.
In Korea, the prevalence of overweight status increased from 26.2% in 1998 to 46.3% in 2021 among men and from 25.1% to 26.9% among women.15 The obesity epidemic has resulted in an increased number of kidney biopsy cases in obese participants. However, the true incidence of obesity-related kidney diseases is still unknown16 because only few reports have studied the incidence according to kidney biopsy results.10,14
The histology of ORG includes glomerulomegaly and FSGS.6,10,14,17 A previous study identifying 128 ORG participants from 6,818 native kidney biopsies found that the mean glomerular diameter in participants with ORG was 1.3-fold larger than that of age and sex-matched normal controls.14 The dominance of perihilar segmental sclerosis might manifest greater ultrafiltration pressure at the afferent vascular pole than at the efferent vascular pole.6 ORG showed increases in the diameter of the afferent arteriole and decreases in podocyte density.6,14,17 These features are caused by increases in renal plasma flow, GFR, filtration fraction, and tubular sodium reabsorption.3 Obesity is a major factor in metabolic syndrome and is associated with the incidence of HTN, DM, hyperuricemia, and dyslipidemia.7,10 Among kidney biopsies, including 239 morbid obese participants (BMI ≥ 40 kg/m2), 27.6% had DMN, and 19.2% had hypertensive nephrosclerosis.10 In our study, we found that obesity was independently associated with an increased prevalence of FSGS and HT-N. However, interestingly, it did not affect ESKD progression. In contrast, obesity was associated with significantly higher risk of ESKD in patients with IgAN and LN in our study indicating the differential effect of obesity according to different types of glomerular diseases. The mechanism of renal injury in immune-mediated GN in obese participants is not clear. Adipose tissue hypertrophy causes an increase in adipokines and inflammatory mediators, such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), leptin, adiponectin, and angiotensinogen, that could play crucial roles in kidney function and BP.18,19 TNF-α and IL-6 were also shown to play a major role in the pathogenesis of systemic lupus erythematosus (SLE).20,21 In addition to heightened inflammation, high blood pressure (BP) in obese participants might also contribute to renal injury, which can activate innate and adaptive immunity and immune cell infiltration in the kidneys.19 Elevated BP is an independent risk factor for disease progression in patients with IgAN and LN.22,23,24 The previous observation showing that higher prevalence not only HTN and proteinuria, but also CKD, dialysis or death events in the overweight or obese IgAN compared to no obese participants also support the negative effect of obesity in IgAN.24 In a study of 393 lupus patients, obesity was independently associated with the incidence of newly developed LN and cumulative organ damage.25 Obesity was related to significant negative effects on function in patients with SLE.26 Given this possible impact of obesity on progression of IgAN and LN, randomized studies testing the effect of weight reduction on outcome are warranted. The prevalence of ORG, defined as FSGS with glomerulomegaly or glomerulomegaly alone, has been reported as 29.4% in morbidly obese participants,10 and this is comparable to the rate in our study (31.5%). However, the spectrum of kidney diseases accompanying ORG was completely different; most kidney diseases were HT-N (56.8%), DMN (25.0%), and acute tubular necrosis (18.2%) in the that study including predominantly white and black races in the United States.10 In contrast, the most common kidney disease accompanying ORG was IgAN (40.9%), followed by DMN (15.2%) and MN (13.5%) in our study. These findings might reflect the regional/racial differences in glomerular disease frequencies.27 We also found that the renal outcomes in participants with ORG accompanied by other renal diseases were significantly worse than those of participants with ORG or other renal diseases alone. This finding suggest that the kidney biopsy is important in predicting outcomes in obese participants. We also found that baseline eGFRs in DMN, IgAN, and LN patients were significantly lower in Group 2 than in Group 3, suggesting that superimposed ORG in these diseases is an important factor in kidney disease progression. Favorable outcomes were seen in participants with ORG alone. Higher serum albumin levels, lower IgA levels, and a history of DM might be considered predictive factors for ORG alone to guide treatment and prognosis in clinical practice.
Despite several novel findings including long-term outcomes, our study had some limitations. First, this study had a retrospective nature, and treatment data were limited. Second, there were no patient BMI results for 31.6% of the total kidney biopsies, so bias could exist in patient selection. Third, among the pathologic data, glomerulomegaly was assessed in routine clinical practice by trained renal pathologists in the tertiary hospital. However, the strength of our study was the large-scale study based on pathologic data. We gathered total 21,697 kidney biopsies from 18 tertiary hospitals in Korea and compared characteristics and long-term outcomes of obese (n = 969) and nonobese (n = 13,522) participants. We analyzed the effect of obesity on risk and prognosis of various glomerular diseases. We also found that many different types of glomerular diseases can be combined with ORG in these participants and these participants might have worse renal outcome. In addition, types of glomerular diseases frequently combined with ORG in obese participants are different according to a different regions and ethnicity. Given that there have been only few reports on pathologic data in severely obese participants in Asia, our study shows the heterogeneity of kidney pathology and outcomes in obese participants and indicates the importance of a kidney biopsy in both deciding the management and predicting outcomes.
ACKNOWLEDGMENTS
We would like to thank all the members of the Korean GlomeruloNephritis Study Group.
Footnotes
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1G1A1005147).
Disclosure: The authors have no potential conflicts of interest to disclose.
Data Sharing Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request. Restrictions apply to the availability of these data. Data are available from the authors with the permission of the data provider and subject to confidentiality agreements.
- Conceptualization: Oh SW.
- Data curation: Kim TB, Ahn SY, Oh J, Bae EH, Chin HJ, Oh SW.
- Formal analysis: Kim TB, Oh SW.
- Investigation: Kim TB, Oh SW.
- Methodology: Kim TB, Chin HJ, Oh SW.
- Supervision: Kim MG, Jo SK, Cho WY, Oh SW.
- Writing - original draft: Oh SW, Kim TB, Jo SK.
- Writing - review & editing: Oh SW, Kim TB, Jo SK.
SUPPLEMENTARY MATERIAL
Risk of obesitya in the mortality of patients with biopsy-proven kidney disease
Clinical characteristics of the obese patients
Risk factors for obesity-related glomerulopathy without other renal diseases
References
- 1.World Health Organization. Obesity and overweight. [Updated 2021]. [Accessed April 27, 2023]. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight .
- 2.Jaacks LM, Vandevijvere S, Pan A, McGowan CJ, Wallace C, Imamura F, et al. The obesity transition: stages of the global epidemic. Lancet Diabetes Endocrinol. 2019;7(3):231–240. doi: 10.1016/S2213-8587(19)30026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stierman B, Afful J, Carroll MD, Chen TC, Davy O, Fink S, et al. National Health and Nutrition Examination Survey 2017–March 2020 prepandemic data files development of files and prevalence estimates for selected health outcomes. [Updated 2021]. [Accessed April 27, 2023]. https://stacks.cdc.gov/view/cdc/106273 . [DOI] [PMC free article] [PubMed]
- 4.Garofalo C, Borrelli S, Minutolo R, Chiodini P, De Nicola L, Conte G. A systematic review and meta-analysis suggests obesity predicts onset of chronic kidney disease in the general population. Kidney Int. 2017;91(5):1224–1235. doi: 10.1016/j.kint.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Chang AR, Chen Y, Still C, Wood GC, Kirchner HL, Lewis M, et al. Bariatric surgery is associated with improvement in kidney outcomes. Kidney Int. 2016;90(1):164–171. doi: 10.1016/j.kint.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman-Edelstein M, et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12(8):453–471. doi: 10.1038/nrneph.2016.75. [DOI] [PubMed] [Google Scholar]
- 7.Salvatore SP, Chevalier JM, Kuo SF, Audia PF, Seshan SV. Kidney disease in patients with obesity: It is not always obesity-related glomerulopathy alone. Obes Res Clin Pract. 2017;11(5):597–606. doi: 10.1016/j.orcp.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Kataoka H, Ohara M, Shibui K, Sato M, Suzuki T, Amemiya N, et al. Overweight and obesity accelerate the progression of IgA nephropathy: prognostic utility of a combination of BMI and histopathological parameters. Clin Exp Nephrol. 2012;16(5):706–712. doi: 10.1007/s10157-012-0613-7. [DOI] [PubMed] [Google Scholar]
- 9.Hong YA, Min JW, Ha MA, Koh ES, Kim HD, Ban TH, et al. The Impact of obesity on the severity of clinicopathologic parameters in patients with IgA nephropathy. J Clin Med. 2020;9(9):2824. doi: 10.3390/jcm9092824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choung HG, Bomback AS, Stokes MB, Santoriello D, Campenot ES, Batal I, et al. The spectrum of kidney biopsy findings in patients with morbid obesity. Kidney Int. 2019;95(3):647–654. doi: 10.1016/j.kint.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Kim SY, Jin DC, Bang BK. Current status of dialytic therapy in Korea. Nephrology (Carlton) 2003;8(Suppl):S2–S9. doi: 10.1046/j.1440-1797.8.s.5.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Jo SK, Ahn SY, Kwon YJ, Lee H, Oh J, et al. Long-term renal outcome of biopsy-proven acute tubular necrosis and acute interstitial nephritis. J Korean Med Sci. 2020;35(26):e206. doi: 10.3346/jkms.2020.35.e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59(4):1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 15.Korea Disease Control and Prevention Agency. The Korea National Health and Nutrition Examination Survey. [Updated 2023]. [Accessed April 27, 2023]. https://knhanes.kdca.go.kr/knhanes/main.do .
- 16.Moon H, Lee Y, Kim S, Kim DK, Chin HJ, Joo KW, et al. Differential signature of obesity in the relationship with acute kidney injury and mortality after coronary artery bypass grafting. J Korean Med Sci. 2018;33(48):e312. doi: 10.3346/jkms.2018.33.e312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuboi N, Okabayashi Y, Shimizu A, Yokoo T. The renal pathology of obesity. Kidney Int Rep. 2017;2(2):251–260. doi: 10.1016/j.ekir.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13(11):633–643. doi: 10.1038/nrendo.2017.90. [DOI] [PubMed] [Google Scholar]
- 19.Hall JE, Mouton AJ, da Silva AA, Omoto AC, Wang Z, Li X, et al. Obesity, kidney dysfunction, and inflammation: interactions in hypertension. Cardiovasc Res. 2021;117(8):1859–1876. doi: 10.1093/cvr/cvaa336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aringer M, Smolen JS. The role of tumor necrosis factor-alpha in systemic lupus erythematosus. Arthritis Res Ther. 2008;10(1):202. doi: 10.1186/ar2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yap DY, Lai KN. The role of cytokines in the pathogenesis of systemic lupus erythematosus - from bench to bedside. Nephrology (Carlton) 2013;18(4):243–255. doi: 10.1111/nep.12047. [DOI] [PubMed] [Google Scholar]
- 22.Contreras G, Pardo V, Cely C, Borja E, Hurtado A, De La Cuesta C, et al. Factors associated with poor outcomes in patients with lupus nephritis. Lupus. 2005;14(11):890–895. doi: 10.1191/0961203305lu2238oa. [DOI] [PubMed] [Google Scholar]
- 23.Ginzler EM, Felson DT, Anthony JM, Anderson JJ. Hypertension increases the risk of renal deterioration in systemic lupus erythematosus. J Rheumatol. 1993;20(10):1694–1700. [PubMed] [Google Scholar]
- 24.Berthoux F, Mariat C, Maillard N. Overweight/obesity revisited as a predictive risk factor in primary IgA nephropathy. Nephrol Dial Transplant. 2013;28(Suppl 4):iv160–iv166. doi: 10.1093/ndt/gft286. [DOI] [PubMed] [Google Scholar]
- 25.Kang JH, Xu H, Choi SE, Park DJ, Lee JK, Kwok SK, et al. Obesity increases the incidence of new-onset lupus nephritis and organ damage during follow-up in patients with systemic lupus erythematosus. Lupus. 2020;29(6):578–586. doi: 10.1177/0961203320913616. [DOI] [PubMed] [Google Scholar]
- 26.Katz P, Yazdany J, Julian L, Trupin L, Margaretten M, Yelin E, et al. Impact of obesity on functioning among women with systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2011;63(10):1357–1364. doi: 10.1002/acr.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Shaughnessy MM, Hogan SL, Thompson BD, Coppo R, Fogo AB, Jennette JC. Glomerular disease frequencies by race, sex and region: results from the International Kidney Biopsy Survey. Nephrol Dial Transplant. 2018;33(4):661–669. doi: 10.1093/ndt/gfx189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of obesitya in the mortality of patients with biopsy-proven kidney disease
Clinical characteristics of the obese patients
Risk factors for obesity-related glomerulopathy without other renal diseases