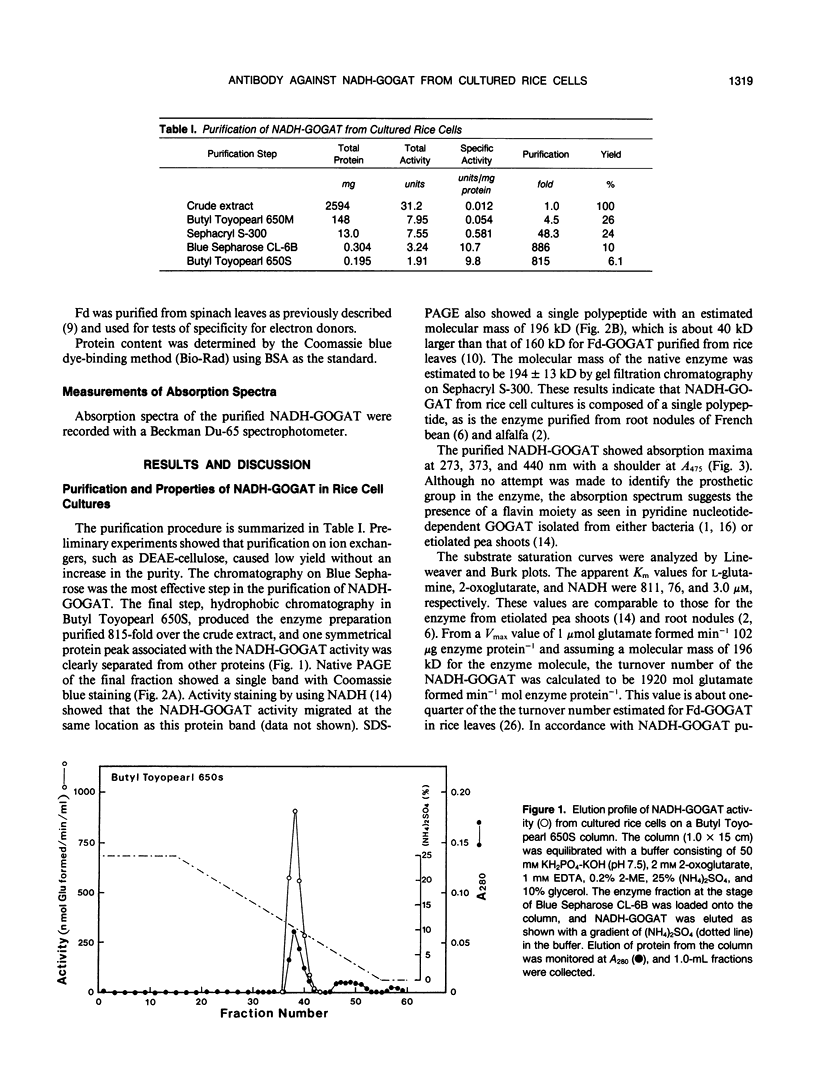
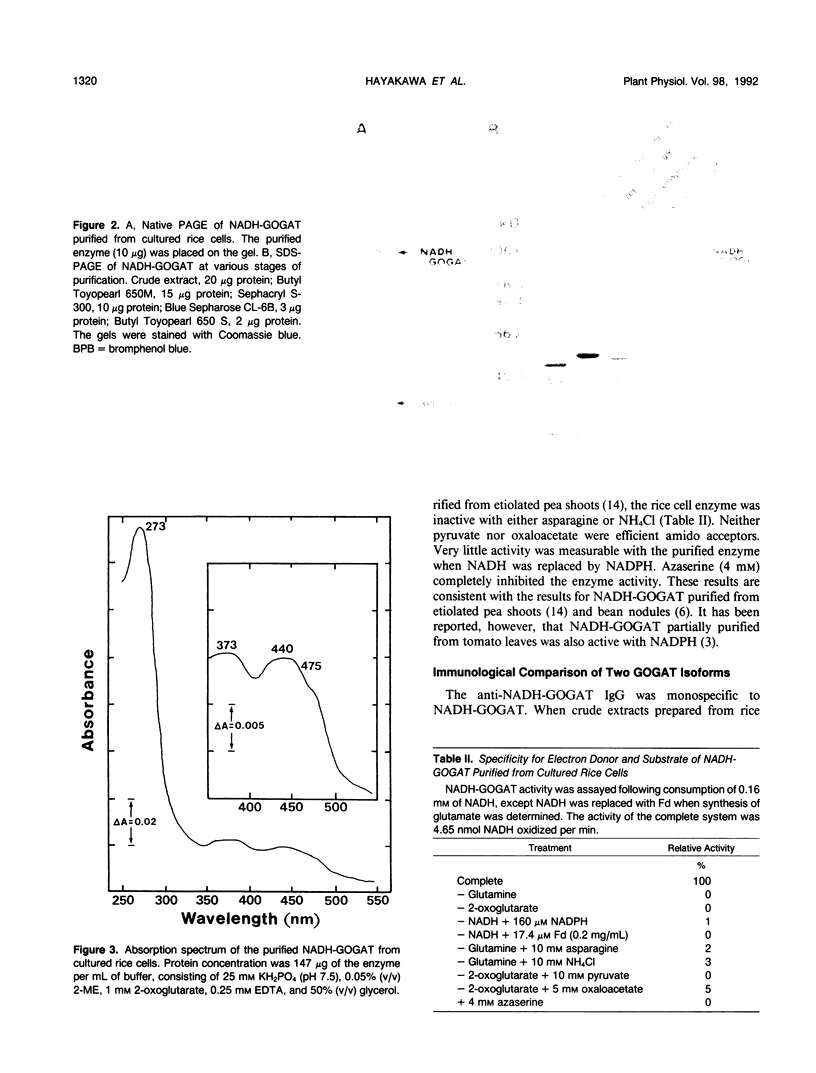
Abstract
To obtain a monospecific antibody against NADH-dependent glutamate synthase (NADH-GOGAT; EC 1.4.1.14), the enzyme was purified to homogeneity from cultured rice cells (Oryza sativa) with column chromatography using Butyl Toyopearl 650M, Sephacryl S-300, Blue Sepharose CL-6B, and Butyl Toyopearl 650S. The specific activity at the final stage of the purification was 9.8 micromoles of glutamate formed per minute per milligram of protein. The yield was 6.1% and purification was 815-fold. Analysis by denaturing gel electrophoresis revealed a single polypeptide with an apparent molecular weight of 196,000, similar to the value of 194,000 estimated for the native protein. Apparent Km values for l-glutamine, 2-oxoglutarate, and NADH were 811, 76, and 3.0 micromolar, respectively. Neither NADPH nor l-asparagine substituted for NADH and l-glutamine, respectively. The enzyme had its absorption maxima at 273, 373, and 440 nanometers with a shoulder at 475 nanometers, suggesting that the rice NADH-GOGAT is a flavoprotein. Monospecific antibody raised against NADH-GOGAT purified from the rice cells was obtained as the first instance for the enzyme in higher plants. Immunological analyses showed that the antibody for rice cell NADH-GOGAT reacted with only the enzyme in extracts from the cells. The anti-NADH-GOGAT antibody did not recognize the ferredoxin-GOGAT purified from rice leaves, and likewise the anti-rice leaf ferredoxin-GOGAT antibody did not react with the NADH-GOGAT purified from the cultured rice cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi K., Suzuki I. Purification and properties of glutamate synthase from Thiobacillus thioparus. J Bacteriol. 1977 Mar;129(3):1173–1182. doi: 10.1128/jb.129.3.1173-1182.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. P., Vance C. P., Heichel G. H., Miller S. S. Purification and Characterization of NADH-Glutamate Synthase from Alfalfa Root Nodules. Plant Physiol. 1989 May;90(1):351–358. doi: 10.1104/pp.90.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila C., Botella J. R., Cánovas F. M., de Castro I. N., Valpuesta V. Different Characteristics of the Two Glutamate Synthases in the Green Leaves of Lycopersicon esculentum. Plant Physiol. 1987 Dec;85(4):1036–1039. doi: 10.1104/pp.85.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella J. R., Verbelen J. P., Valpuesta V. Immunocytolocalization of Ferredoxin-GOGAT in the Cells of Green Leaves and Cotyledons of Lycopersicon esculentum. Plant Physiol. 1988 May;87(1):255–257. doi: 10.1104/pp.87.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F. L., Cullimore J. V. Two Isoenzymes of NADH-dependent Glutamate Synthase in Root Nodules of Phaseolus vulgaris L: Purification, Properties and Activity Changes during Nodule Development. Plant Physiol. 1988 Dec;88(4):1411–1417. doi: 10.1104/pp.88.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. W., Coruzzi G. M. Photorespiration and light act in concert to regulate the expression of the nuclear gene for chloroplast glutamine synthetase. Plant Cell. 1989 Feb;1(2):241–248. doi: 10.1105/tpc.1.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi K., Yamaya T., Mae T., Ojima K. A Role for Glutamine Synthetase in the Remobilization of Leaf Nitrogen during Natural Senescence in Rice Leaves. Plant Physiol. 1991 Jun;96(2):411–417. doi: 10.1104/pp.96.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. E., Stadtman E. R. Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J Biol Chem. 1972 Nov 25;247(22):7407–7419. [PubMed] [Google Scholar]
- Morris P. F., Layzell D. B., Canvin D. T. Ammonia Production and Assimilation in Glutamate Synthase Mutants of Arabidopsis thaliana. Plant Physiol. 1988 May;87(1):148–154. doi: 10.1104/pp.87.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H., Watanabe M., Hase T., Sugiyama T. Molecular cloning and characterization of complementary DNA encoding for ferredoxin-dependent glutamate synthase in maize leaf. J Biol Chem. 1991 Feb 5;266(4):2028–2035. [PubMed] [Google Scholar]
- Sakamoto A., Ogawa M., Masumura T., Shibata D., Takeba G., Tanaka K., Fujii S. Three cDNA sequences coding for glutamine synthetase polypeptides in Oryza sativa L. Plant Mol Biol. 1989 Nov;13(5):611–614. doi: 10.1007/BF00027323. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Audet C., Oaks A. Influence of light on the ferredoxin-dependent glutamate synthase in maize leaves. Plant Physiol. 1987 Jul;84(3):578–581. doi: 10.1104/pp.84.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Gadal P. Glutamate synthase from rice leaves. Plant Physiol. 1982 Apr;69(4):848–852. doi: 10.1104/pp.69.4.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Vidal J., Gadal P. Glutamate synthase isoforms in rice: immunological studies of enzymes in green leaf, etiolated leaf, and root tissues. Plant Physiol. 1982 Sep;70(3):827–832. doi: 10.1104/pp.70.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey S. V., Tsai F. Y., Edwards J. W., Walker E. L., Coruzzi G. M. Chloroplast and cytosolic glutamine synthetase are encoded by homologous nuclear genes which are differentially expressed in vivo. J Biol Chem. 1988 Jul 15;263(20):9651–9657. [PubMed] [Google Scholar]





