Abstract
The recently discovered deep-sea Capelinhos hydrothermal edifice, ~ 1.5 km of the main Lucky Strike (LS) vent field (northern Mid-Atlantic Ridge), contrasts with the other LS edifices in having poorly-altered end-member hydrothermal fluids with low pH and chlorine, and high metal concentrations. Capelinhos unique chemistry and location offer the opportunity to test the effects of local abiotic filters on faunal community structure while avoiding the often-correlated influence of dispersal limitation and depth. In this paper, we characterize for the first time the distribution patterns of the Capelinhos faunal communities, and analyze the benthic invertebrates (> 250 µm) inhabiting diffusive-flow areas and their trophic structures (δ13C, δ15N and δ34S). We hypothesized that faunal communities would differ from those of the nearest LS vent edifices, showing an impoverished species subset due to the potential toxicity of the chemical environment. Conversely, our results show that: (1) community distribution resembles that of other LS edifices, with assemblages visually dominated by shrimps (close to high-temperature focused-fluid areas) and mussels (at low-temperature diffuse flow areas); (2) most species from diffuse flow areas are well-known LS inhabitants, including the bed-forming and chemosymbiotic mussel Bathymodiolus azoricus and (3) communities are as diverse as those of the most diverse LS edifices. On the contrary, stable isotopes suggest different trophodynamics at Capelinhos. The high δ15N and, especially, δ13C and δ34S values suggest an important role of methane oxidation (i.e., methanotrophy), rather than the sulfide oxidation (i.e., thiotrophy) that predominates at most LS edifices. Our results indicate that Capelinhos shows unique environmental conditions, trophic structure and trophodynamics, yet similar fauna, compared to other LS edifices, which suggest a great environmental and trophic plasticity of the vent faunal communities at the LS.
Subject terms: Biodiversity, Community ecology
Introduction
Understanding the processes structuring faunal assemblages is at the core of community ecology1. Regional species pools are determined by speciation, extinction and dispersion interacting with smaller-scale abiotic and biotic processes that ultimately determine species coexistence and community structure at local habitats2–4. Assessing these community assembly processes is key to predict disturbance impacts on, and resilience of, ecosystems5.
In 1977, lush taxon-novel communities fueled by in situ microbial chemoautotrophy were discovered in the Galapagos Rift hydrothermal vents at 2550 m depth6,7. Deep-sea vents are often dominated by large foundation chemosymbiotic species not found elsewhere, which promote the establishment of smaller invertebrates8,9. Overall, this gives rise to highly productive, biomass-rich communities, orders of magnitude denser than those of the surrounding, energy-limited deep sea (reviewed in10–12). More than four decades of exploration have revealed hundreds of active vent fields patchily distributed along diverse tectonic settings in the world oceans13. However, all together these numerous island-like habitats occupy only 50 km2 worldwide14. Vents are thus small natural features with a biogeochemical and ecological relevance disproportionate to their size14. On top of that, our knowledge of the complex processes driving community assembly in vent ecosystems is still insufficient11. This knowledge gap is worrisome given the current context where climate change, pollution and deep-sea mining could potentially trigger adverse impacts on vents habitats in the near future15–17.
Vent communities differ across tectonic settings, constituting different biogeographic provinces covering entire, or parts of, ocean basins10,18–20. Regionally, larval dispersal connects the network of island-like vent habitats separated by 10–100 s of kilometers, but topographic barriers and oceanic circulation regimes influence connectivity21–26. At a more local scale, vent fields rarely harbor all province species, and significant faunal dissimilarities have been observed even between neighboring sites27–30. Differences may be driven by local environmental filters, such as distinct fluid chemistry and substratum nature28,29,31,32 and/or by intricate biological processes33–36. More complex patterns occur in arc and back-arc basin vents (e.g.,30,37) and at slower-spreading mid-oceanic ridges (e.g.,27,38) where geology is more heterogeneous.
In the slow-spreading northern Mid-Atlantic Ridge (nMAR) (Fig. 1A), visually-dominant species assemblages differ between vent fields, creating a complex community mosaic along the ridge27,28,39–41. Generally, shrimps dominate higher-temperature habitats. Mirocaris fortunata (Martin & Christiansen, 1995) dominates the Moytirra, Menez Gwen and Lucky Strike vents, and Rimicaris exoculata Williams & Rona, 1986 dominates deeper vents such as TAG, Snake Pit or Rainbow28,40,42. Intermediate temperature habitats appear to be dominated by newly described gastropod assemblages including two different species: Lepetodrilus atlanticus Warén and Bouchet 2001 at Menez Gwen and Peltospira smaragdina Warén and Bouchet 2001 at seven other deeper vent fields43. At lower-temperature habitats, chemosymbiotic mussels are the main foundation species. The shallower Menez Gwen and Lucky Strike vent fields (850–1700 m depth) are dominated by Bathymodiolus azoricus Cosel & Comtet, 1999, which is less abundant at the deeper and ultramafic-hosted Rainbow field (2300 m depth)28,44. Broken Spur (3100 m depth) is a hybridizing zone between B. azoricus and Bathymodiolus puteoserpentis Cosel, Métivier & Hashimoto, 1994 the latter exclusively dominating Snake Pit (3500 m) and Logatchev (3000 m depth) fields27,40,45. At TAG (3670 m depth), the metal-enriched fluids are hypothesized to prevent mussel establishment27,46. Mussels are also absent at Moytirra (2900 m depth), dominated by the gastropod P. smaragdina Warén & Bouchet 2001, and Ashadze-1 (4200 m depth)42,47. Adding more complexity to this mosaic, Lost City ultramafic-hosted vents (800 m, ~ 15 km off axis) do not support the typical dense vent shrimp/mussel assemblages48, although evidence suggest it did so in the past49,50. Given this multifaceted picture and despite the 46 years of vent research, the processes driving the community assembly at nMAR vents are just starting to be resolved.
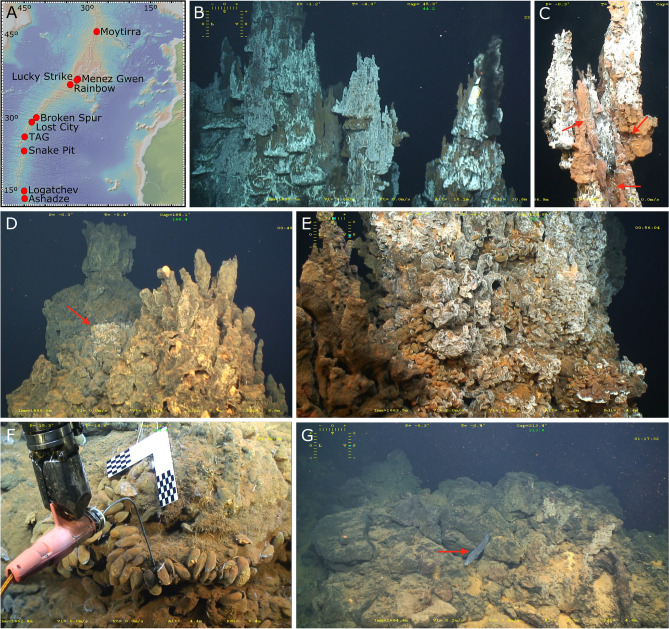
Figure 1.
(A). Main hydrothermal fields in the northern Mid-Atlantic Ridge; Capelinhos is located ~ 1.5 km off the main Lucky Strike field. Capelinhos habitats. (B). Active and inactive black smokers with microbial mats at the summit of the structure. (C). Shrimp aggregations (red arrows) probably belonging to Mirocaris fortunata on top of a black smoker. (D). Top of a senescent chimney with a patch of Bathymodiolus azoricus mussels (red arrow) in between its two heads. (E). Dense B. azoricus beds and microbial mats at the base of a senescent chimney. F. Patch of large B. azoricus with sparse shrimps; the CHEMINI analyzer inlet and our checkerboard calibrated target are visible. (G). Cataetyx-like fish (red arrow) on sulphide rubble surroundings. Map made with GeoMapApp 3.6.15 (www.geomapapp.org)/CC BY/CC BY (Ryan et al.51).
Here, we characterize for the first time the distribution, composition and trophic structure of the Capelinhos vent communities, particularly those associated to mussel beds, and compare them to those of other Lucky Strike (LS) edifices and to several nMAR vents. Capelinhos is a recently-discovered, isolated hydrothermal structure located off the main LS field (nMAR) (Fig. 1A)52. Capelinhos emits poorly-altered end-member fluids with low pH and chlorine, and high metal concentrations. In contrast, the other LS edifices show intermediate (e.g., Eiffel Tower, Montségur) or highly-altered fluids (e.g., Crystal) with higher pH and lower metal concentrations52,53. The proximity and similar depth of Capelinhos to other LS edifices create a natural experiment restricting the effects of dispersal limitation and depth-related processes while isolating the putative influence of local abiotic conditions on faunal communities. We hypothesize that the potentially harsher chemistry, i.e., low pH and high metal concentrations, of Capelinhos could act as environmental filter, influencing the fauna distribution and composition, and preventing the establishment of some species, compared to other LS edifices.
Results
Habitat characterization based on ROV imagery
Capelinhos edifice has a steep profile with two main structures (Fig. 1, Supplementary material Video S1). The first structure has several “candelabra-like” chimneys, some emitting highly-focused hydrothermal fluids (Fig. 1B,C, Supplementary material Video S1–1:30 min). These chimneys are densely colonized by white microbial mats and sparse shrimp aggregations (probably M. fortunata) close to the focused fluids (Fig. 1C, Supplementary material Video S1–5:00 min). Dense mussel aggregations (including patches of juveniles) and microbial mats occur at the base. The second structure is a single, large, senescent Y-shape chimney. The limited diffuse-flow area found at the top is colonized by a small patch of large mussels (Fig. 1D, Supplementary material Video S1–7:13 min). The base of this structure is colonized by dense mussel beds (covered or not by microbial mats), especially on one of its sides (Fig. 1E, Supplementary material Video S1–8:40 min). The few flanges at the base of each main structure are colonized by large mussels and shrimps (Fig. 1F, Supplementary material Video S1). Crabs, probably Segonzacia mesatlantica (Williams, 1988), and shrimps were present in between and over the mussels (Fig. 1F, Supplementary material Video S1–9:36). Some fishes occur at the vicinity of the chimneys and in peripheral areas (Fig. 1G, Supplementary material Video S1–10:00 min).
Environment, community composition and species diversity
Temperatures over mussel assemblages range from 4.32 to 9.88 °C (Table 1 and Supplementary material Fig. S1). Between-sample variability is relatively high, despite the close proximity of the probes (~ 50 cm), as observed for Fe(II) (0.98–2.40 µM) and even more for ƩS (21.80–44.8 µM) (Table 1; Supplementary material Table S2 and Fig. S1). Compared with the habitats colonized by B. azoricus at the Eiffel Tower edifice, the temperatures at Capelinhos are similar to those in cold and intermediate-temperature habitats, while the high Fe(II) and, especially, ƩS concentrations are more similar to those of the warm habitats, highlighting Capelinhos distinct fluid chemistry (Table 1, Supplementary material Table S2 and Fig. S2).
Table 1.
Temperature (T, °C), iron (Fe(II), µM) and sulfide (ƩS, µM) concentrations (mean ± standard deviation) in Bathymodiolus azoricus mussel bed samples at Capelinhos.
| T | Fe(II) | ƩS | ||||
|---|---|---|---|---|---|---|
| Mean | Min | Max | ||||
| Capelinhos | 5.68 ± 0.67 | 4.32 | 9.88 | 1.77 ± 0.58 | 31.88 ± 9.47 | |
| Eiffel Tower | Cold | 5.11 ± 0.37 | NA | NA | 0.54 ± 0.37 | 3.31 ± 1.94 |
| Intermediate | 6.04 ± 0.59 | NA | NA | 1.13 ± 0.98 | 12.83 ± 6.28 | |
| Warm | 7.59 ± 1.97 | NA | NA | 3.33 ± 2.36 | 32.38 ± 19.16 | |
| Montségur | 5.2 | 4.6 | 6.1 | 0.2 ± 0.1 | 2.7 ± 0.2 | |
| 6.9 | 5.1 | 11.5 | 1.1 ± 0.3 | 3.1 ± 1 | ||
| 9.5 | 6.1 | 22.1 | 2.2 ± 0.2 | 2.3 ± 0.2 | ||
| 5.5 | 5.1 | 11.4 | 0.2 ± 0.1 | 3.2 ± 2.7 | ||
| 5.3 | 4.6 | 7.1 | 0.6 ± 1.1 | 0.9 ± 0.2 | ||
Environmental information of B. azoricus cold, intermediate and warm microhabitats of the Eiffel Tower44 edifice and samples from Montségur edifice54 (both located at Lucky Strike) are provided for comparison. Mean temperature associated to Fe(II) and ƩS measurements at Capelinhos were 4.75 ± 0.57 and 5.04 ± 0.30 °C, respectively (see raw data in Supplementary material Table S2 and Fig. S2).
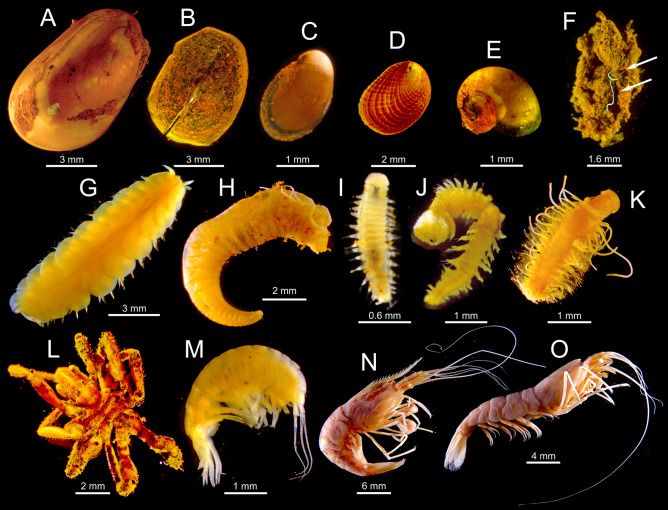
We have identified 1986 individuals from 28 species/morphospecies representing five phyla (Table 2, Fig. 2, Supplementary material Table S3). Genetic analyses confirm the dominant species as belonging to B. azoricus (https://sextant.ifremer.fr/Donnees/Catalogue#/metadata/72f13a1b-3770-4108-828c-b12aa4249987). Following dominant species are the tubicolous annelid Amphisamytha lutzi (Desbruyères & Laubier, 1996) and dirivultid copepods (Table 1; Fig. 2). The annelid Branchipolynoe seepensis Pettibone, 1986 and the nematode Oncholaimus dyvae Zeppilli et al., 2019 were also abundant, often occurring inside B. azoricus and within A. lutzi matrix tubes, respectively (Fig. 2B,G).
Table 2.
Relative mean (± sd) abundance (N) and isotopic composition (δ13C, δ15N and δ34S) of species found at the Capelinhos hydrothermal structure, 1.5 km off the Lucky Strike vent field (nMAR).
| Phylum | Class | Order | Species | N | δ13C | δ15N | δ34S | ET |
|---|---|---|---|---|---|---|---|---|
| Annelida | Polychaeta | Terebellida | Amphisamytha lutzi | 18.76 ± 2.28 | − 17.16 ± 1.60 (15) | 3.07 ± 1.57 (15) | 11.26 ± 1.05 (15) | S |
| Phyllodocida | Glycera tesselata | 0.03 ± 0.05 | − 21.84 | 9.11 | – | S | ||
| Branchipolynoe seepensis | 8.91 ± 4.76 | − 20.75 ± 3.04 (6) | − 3.10 ± 3.71 (6) | 8.71 ± 0.42 (6) | S | |||
| Branchipolynoe sp. 2 | 0.12 ± 0.20 | − 19.18 | − 1.15 | 9.72 | – | |||
| Polynoidae sp. 3 | 0.48 ± 0.50 | – | – | – | – | |||
| Branchinotogluma sp. | 0.47 ± 0.15 | − 23.61 ± 6.89 (3) | 6.68 ± 1.06 (3) | 10.23 ± 0.98 (3) | – | |||
| Branchinotogluma sp. 2 | 0.03 ± 0.05 | – | – | – | – | |||
| Hesionidae sp. | 0.20 ± 0.35 | – | – | – | – | |||
| Eunicia | Ophryotrocha fabriae | 2.56 ± 0.96 | − 24.88 | 2.55 | – | S | ||
| Arthropoda | Hexanauplia | Harpacticoida | Smacigastes micheli | 0.30 ± 0.30 | – | – | – | S |
| Ameiridae sp. 1 | 5.78 ± 5.09 | – | – | – | M | |||
| Miraciidae sp. | 3.47 ± 4.37 | – | – | – | M | |||
| Siphonostomatoida | Dirivultidae sp. | 18.21 ± 3.24 | − 19.37 ± 0.16 (2) | 0.75 ± 0.36 (2) | 9.40 ± 0.38 (2) | M | ||
| Malacostraca | Amphipoda | Luckia striki | 0.09 ± 0.15 | − 20.18 | 4.46 | – | S | |
| Bouvierella curtirama | 0.65 ± 0.57 | − 19.06 ± 0.01 (2) | 2.80 ± 0.51 (2) | 10.91 ± 2.30 (2) | S | |||
| Amphipod sp. 1 | 0.03 ± 0.05 | − 18.75 | 3.27 | – | – | |||
| Decapoda | Mirocaris fortunata | 2.85 ± 3.10 | − 16.72 ± 1.75 (10) | 6.34 ± 0.97 (10) | 10.34 ± 1.71 (10) | S | ||
| Alvinocaris markensis | 0.10 ± 0.17 | − 23.47 | 2.79 | 8.55 | S | |||
| Ostracoda | Ostracoda sp. | 2.68 ± 1.35 | – | – | – | – | ||
| Pycnogonida | Pantopoda | Sericosura sp. | 0.20 ± 0.35 | − 21.57 | 4.61 | 10.35 | M | |
| Mollusca | Bivalvia | Mytilida | Bathymodiolus azoricus | 21.90 ± 3.17 | − 20.95 ± 3.08 (6) | − 4.70 ± 2.53 (6) | 7.97 ± 1.66 (6) | S |
| Gastropoda | Lepetellida | Lepetodrilus atlanticus | 1.50 ± 1.33 | − 20.04 | 4.30 | 12.17 | S | |
| Pseudorimula midatlantica | 1.81 ± 1.04 | − 25.99 ± 0.51 (2) | 2.28 ± 0.04 (2) | 8.05 ± 0.08 (2) | S | |||
| Trochida | Protolira valvatoides | 0.77 ± 0.67 | − 22.98 | 3.97 | 11.97 | S | ||
| Lurifax vitreus | 0.03 ± 0.05 | – | – | – | S | |||
| Cycloneritida | Divia briandi | 0.29 ± 0.50 | − 11.89 | 5.95 | 8.29 | S | ||
| Nematoda | Enoplea | Enoplida | Oncholaimus dyvae | 7.16 ± 4.40 | − 18.32 | 6.32 | 9.51 | S |
| Nemertea | Nemertea sp. | 0.63 ± 0.76 | − 17.23 | 2.04 | 10.85 | – |
ET column indicates if the species (S) or morphotype (M) is found at Eiffel Tower edifice in the main Lucky Strike (Alfaro-Lucas et al.55; J.M. Alfaro-Lucas pers. obs.). In brackets the number of samples used to estimate the isotopic composition of species.
Figure 2.
Main macrobenthic invertebrate species inhabiting Capelinhos. (A) Bathymodiolus azoricus. (B) Pseudorimula midatlantica. (C) Lepetodrilus atlanticus. (D) Divia briandi. (E) Protolira valvatoides. (F) Oncholaimus dyvae (arrow) within A. lutzi tube. (G) Branchipolynoe seepensis. (H) Amphisamytha lutzi. (I) Ophryotrocha fabriae. (J) Glycera tesselata. (K) Hesionidae sp. (L) Sericosura sp. (M) Bouvierella curtirama. (N) Alvinocaris markensis. (O) Mirocaris fortunata.
0D, 1D and 2D are as high at Capelinhos as at the assemblages of B. azoricus from Eiffel Tower and Montségur edifices (Fig. 3). βsim is equal to 0 when compared to the LS vents. In other words, their assemblages do not differ in species composition from those observed at the LS (AU-P = 100) (Fig. 4). Capelinhos and LS cluster with the shallower Menez Gwen (850 m depth), whereas deeper vents, including Rainbow (2300 m), cluster together (Fig. 4) highlighting main faunal composition differences between MAR shallower and deeper vents.
Figure 3.
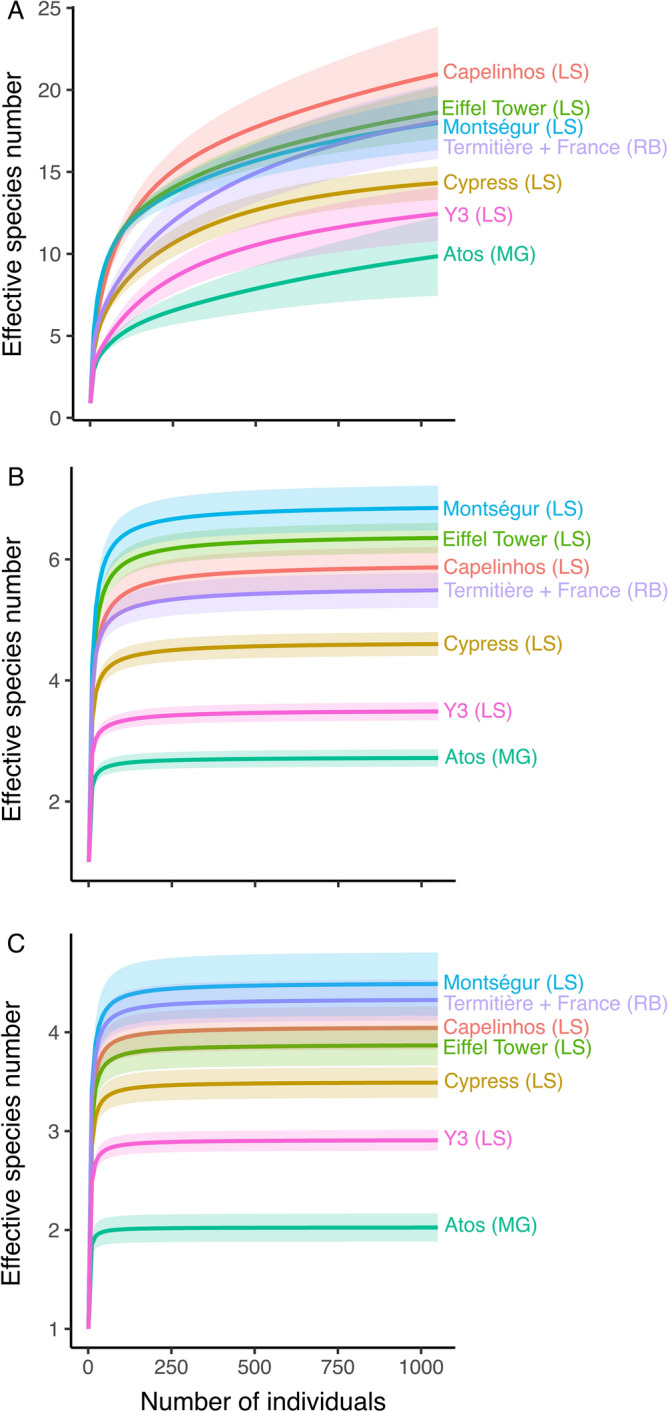
Rarefaction curves and 95% confidence intervals (shaded areas) based on the abundance of the communities at different hydrothermal edifices on the northern Mid-Atlantic Ridge. (A) Species richness. (B) Shannon index. (C) Simpson index. LS, Lucky Strike; MG, Menez Gwen and RB, Rainbow vent fields. LS, MG and RB data from Sarrazin et al.44.
Figure 4.
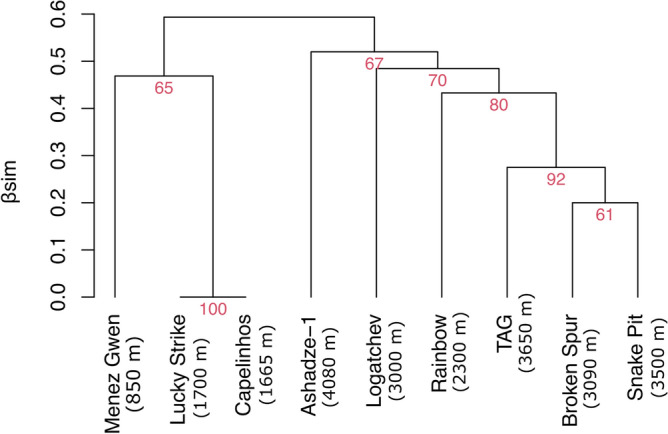
Hierarchical cluster analysis of vent communities at hydrothermal fields and Capelinhos edifice (off the main Lucky Strike vent field) on the northern Mid-Atlantic Ridge. Red numbers: Approximately Unbiased p value. Data modified from Boschen-Rose and Colaço41.
Stable isotopes
Among Capelinhos fauna, mean δ13C is more negative in the limpet Pseudorimula midatlantica J. H. McLean, 1992 and less negative in the gastropod Divia briandi (Warén & Bouchet, 2001) (Table 1, Fig. 5A). Mean δ15N is lower in B. azoricus and higher in the polychaete Glycera tesselata Grube, 1863 (Table 1, Fig. 5A). Mean δ34S is also lower in B. azoricus and higher in the limpet L. atlanticus (Table 1, Fig. 5B). The δ13C/δ15N isotopic structure show a more-compact, rhomb-like structure (B. azoricus and B. seepensis at the bottom, the predator G. tesselata at the top, and the grazers P. midatlantica and D. briandi in the left and right corners, respectively) when compared to the common “upper diagonal” one of Eiffel Tower (B. azoricus and B. seepensis at the bottom and Mirocaris fortunata and Nemertea sp. on the top) (Fig. 5A). Mean δ13C and δ34S are significantly higher (δ13C: t = 3.93, DF = 36, P < 0.001; δ34S: t = 8.65, DF = 27, P < 0.001) at Capelinhos than at Eiffel Tower (Fig. 5A,B).
Figure 5.
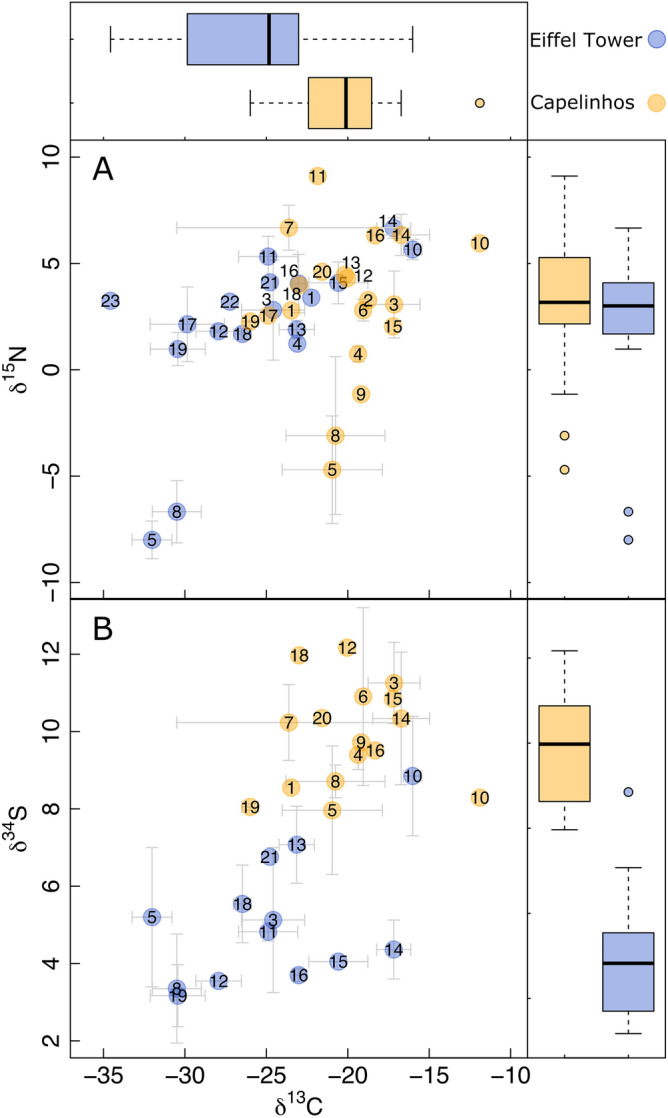
Isotopic composition of species found in Capelinhos structure and Eiffel Tower edifice (Lucky Strike vent field). (A) δ13C/δ15N isotopic space. (B) δ13C/δ34S isotopic space. Boxplots show the δ13C (top), δ15N (top right) and δ34S (bottom right) isotopic value distributions. 1 = Alvinocaris markensis, 2 = Amphipoda sp., 3 = Amphisamytha lutzi, 4 = Dirivultidae sp., 5 = Bathymodiolus azoricus, 6 = Bouvierella curitama, 7 = Branchinotogluma sp., 8 = Branchipolynoe seepensis, 9 = Branchipolynoe sp., 10 = Divia briandi, 11 = Glycera tesselata, 12 = Lepetodrilus atlanticus, 13 = Luckia striki, 14 = Mirocaris fortunata, 15 = Nemertea sp., 16 = Oncholaimus dyvae, 17 = Ophryotrocha fabriae, 18 = Protolira valvatoides, 19 = Pseudorimula midatlantica, 20 = Sericosura sp., 21 = Lirapex costellata, 22 = Pseudorimula midatlantica, 23 = Smacigastes micheli. Eiffel Tower data from Alfaro-Lucas et al.56.
Discussion
Contrary to our expectations, our results suggest a negligible role of the putatively more toxic chemistry of Capelinhos in the distribution and structure of faunal communities compared to those at other LS edifices. The distribution of assemblages along the hydrothermal gradient match the archetypal distribution observed in LS. For instance, shrimps, likely M. fortunata, where observed at the warmest habitats close to focused fluids, whereas B. azoricus beds were observed at lower-temperature, fluid-diffusive habitats (4.32–9.88 °C), as described for the Eiffel Tower edifice44,57–60. All species found at Capelinhos lower temperature diffusive-fluid habitat, including several taxa identified to genus level and/or as morphotypes (Alfaro-Lucas pers. obs.), inhabit the other LS edifices, sharing the dominance of B. azoricus28,39,59,61. Species abundances also suggest a similar assemblage structure at Capelinhos and at the LS Eiffel Tower and Montségur edifices44,54,55,59. Specifically, the dominance of B. azoricus, B. seepensis and A. lutzi, as observed at Capelinhos, is indicative of lower-temperature mussel-bed habitats in the Eiffel Tower edifice59.
The putative harsher chemical environment linked to Capelinhos fluids does not limit species diversity at mussel beds, as hypothesized, being instead comparable to those in the most diverse LS edifices44, as supported by the rarefied diversities. Similar results have been reported in mussel beds and tubeworm bushes on the East Pacific Rise, where the assemblages from different chemical environments exhibit similar diversities and community structures62,63. At LS, B. azoricus and its associated species colonize lower temperature-habitats within edifices with different chemistries, highlighting their environmental44 and trophic64,65 plasticity. However, iron concentrations in the end-member fluid at Capelinhos are the highest of LS (2789.4 ± 84.8 μM)52,53, leading to higher iron concentrations in the mixing gradient between seawater and hydrothermal fluid at Capelinhos than to other LS sites66,67. Very diluted environments characterized with strong lateral entrainment of seawater are trickier to characterize. Nevertheless, chemical analyses in our study revealed that Fe(II) and ƩS concentrations in cooler diffuse flow areas tend to be higher in Capelinhos than at similar areas with similar temperature and similar mussel assemblages at Eiffel Tower and Montségur edifices (LS)54,59. High metal concentrations are hypothesized to limit and even prevent colonization by the species of Bathymodiolus at some nMAR vents27. However, our results suggest that neither B. azoricus, nor the associated fauna, are limited at Capelinhos lower temperature-habitats, highlighting their ability to colonize a wide range of chemical environments. The complex 3D biogenic habitat created by B. azoricus likely promotes small scale turbulence and enhances fluid dilution with seawater, buffering against harsh and concentrated chemical environment and fostering a similarly structure of associated fauna44, as observed for other vent foundation species elsewhere9,62,63. The mechanisms at play are unclear, but in addition to altering environmental conditions and fluid flow, mussels could increase the habitat complexity, niche availability and productivity, among others8,9,63,68.
The Rainbow field (2300 m depth, ~ 200 km south from Capelinhos) also shows high concentrations of iron in end-member fluids [up to 24,050 Fe(II) (µM)]69, and a limited abundance of B. azoricus, which are covered with orange metal deposits as observed at Capelinhos (J. Sarrazin pers. comm.). However, species similarity was higher between Capelinhos, LS and Menez Gwen (800 m depth, ~ 100 km from LS) than between Capelinhos and Rainbow, which agrees with recently results showing higher similarity between LS and Menez Gwen41. This result could suggest that processes acting at broader scales, such as species dispersal limitation shaping the available colonizing species, may play a stronger role in governing the species composition of mussel assemblages along the ridge44 than high iron concentrations27. In fact, the species diversity of B. azoricus assemblages at Rainbow is similar to those from the richest LS edifices, including Capelinhos, reinforcing the idea that high metal concentration alone may not necessarily lead to a decrease in species diversity.
Despite the similar faunal composition and structure, stable isotopes reveal profound trophodynamic differences between Capelinhos and other LS edifices. nMAR vent communities usually present an upward-diagonal-like δ13C/δ15N structure, with two rather independent compartments: (1) B. azoricus and B. seepensis (with the most negative δ13C/δ15N isotopic values) being isolated in one end, and (2) bacterivores, detritivores, scavengers and predators (with less negative δ13C and positive δ15N isotopic values) being spread at the other extreme (e.g.,55,64,65,70). Conversely, Capelinhos shows: (1) a rhombus-like δ13C/δ15N structure, with B. azoricus and B. seepensis at the bottom, the predator G. tesselata at the top, and the grazers P. midatlantica and D. briandi in the left and right corners, respectively; (2) a not so evident isolation of B. azoricus and B. seepensis because dirivultid copepods and the Branchipolynoe found outside their hosts showed closed isotopic values, and (3) an overall less negative δ13C and more positive δ15N isotopic values, particularly in B. azoricus and B. seepensis. Notable exceptions are the shrimps A. markensis and M. fortunata that showed similar δ13C/δ15N isotopic composition between edifices.
Less negative δ13C values may be indicative of the reductive tricarboxylic acid (rTCA) cycle rather than the Calvin–Benson–Bassham (CBB) cycle65. However, rTCA is usually associated with vigorous-fluid flux rather than lower temperature habitats65. Furthermore, less negative δ13C and more positive δ15N ratios have been also observed for B. azoricus at Sintra, a LS edifice where methane is available in higher concentrations71. This suggests that methane oxidation (methanotrophy) could be an important energetic pathway at Capelinhos, in addition to CCB (thiotrophy). Although not as evident as in our study, Rainbow assemblages of B. azoricus show a similar rhombus-like δ13C/δ15N structure, less negative δ13C and more positive δ15N isotopic values, which have been also attributed to the higher contribution of methanotrophy65,69.
The δ34S values of Capelinhos fauna, more positive than that of Eiffel Tower, also support the methanotrophy hypothesis. Values over 10‰ are usually associated to consumption of photosynthetic organic matter72. However, this source has been consistently discarded in nMAR vent food webs70, including those at LS55,64,65. Furthermore, Capelinhos is roughly at the same depth and only ~ 1.5 km apart from the other LS edifices. This and the overall low δ15N (− 4.70 to 9.11‰) allows discarding a depth/location effect on a putative increase in photosynthetic organic matter suggesting instead chemosynthetic pathways. Methanotrophy instead of thiotrophy may lead to more positive δ34S values73 and Capelinhos fluids are naturally enriched in 34S, reflecting contrasting subseafloor fluid/rock interactions compared to the other LS edifices74. Thus, we suggest the more positive δ34S values at Capelinhos are driven by methanotrophy and geological processes influencing fluid composition. Further geochemical and microbiological studies are required to disentangle these intriguing isotopic results, including the measurement of Capelinhos methane concentrations in both end-member and diffuse fluids, and evaluation of the composition of microbial communities compared to those found at other LS edifices. Broadly, our isotopic results: (1) highlight the necessity of characterizing isotopic sources while analyzing multiple isotopes to confidently interpret trophodynamics at chemosynthetic-based habitats, and (2) support the hypothesis that B. azoricus and its associated fauna do not depend on specific trophic pathways or food sources, but rather have a great trophic plasticity allowing them to colonize a wide array of contrasting chemical environments, as previously postulated44,65,75,76.
Material and Methods
Study area, sampling and sample processing
Capelinhos is located ~ 1.5 km to the east of the main Lucky Strike (LS) area (nMAR) at 1665 m depth (37.28917 N, − 32.26388 E)52 (Fig. 1A) (not to be confused with the Capelinhos volcano on the Faial Island, Azores Archipelago77). The LS hydrothermal vent field (~ 1700 m depth), discovered in 1992, is located on the Lucky Strike Seamount on the Azores Triple Junction61,78. It is a basalt-hosted vent field fueled by a magmatic chamber located at 3–3.5 km depth78,79. Hydrothermal activity occurs at > 20 sulfide edifices/structures located around a fossil lava lake of ~ 300 m in diameter situated in between three ancient volcanic cones78,79. Edifices emit both high-temperature focused-fluids, ranging from 200 to 340 °C, and low-temperature diffuse venting, and show distinct fluid chemical compositions52,53. Nevertheless, evidence suggest a common reaction zone for the entire field, including Capelinhos53.
Capelinhos is formed by a 20-m high mound discovered in 2009 in the microbathymetric data during the Bathyluck 2009 cruise (10.17600/9030040) 52. Hydrothermal activity was only confirmed in 2013, when Capelinhos was visually inspected during the Momarsat 2013 cruise (10.17600/13030040) 52. Hydrothermal activity is mainly localized at the mound top, where inactive and active “candelabra”-like chimneys (~ 12 m high) vent focused high-temperature hydrothermal fluids (T = 324 °C) whereas diffuse venting areas are scarce and mainly occur at the mound base52,53. Compared to nearby LS vent edifices, Capelinhos shows poorly-altered fluids that are quickly transported from the common LS reaction zone to the seafloor, giving rise to a fluid end-member chemistry with very low pH (2.56) and chloride (262.3 ± 0.1 mM), and high iron (2789.4 ± 84.8 μM) and manganese (639.5 ± 27.6 μM) concentrations (see Table 2 in53).
Capelinhos was revisited during the Momarsat 2014 (10.17600/14000300) and 2015 (10.17600/15000200) cruises on board of the R/V Pourquoi Pas? which visually inspected chimneys, diffuse flow areas and the periphery using the ROV Victor6000. In 2014, three temperature probes were deployed on a large-size mussel bed on a diffuse flow area at the base of one edifice. The probes registered temperatures every 15 min for 9 months, form the 24th July 2014 to the 20th/23rd April 2015. In 2015, prior to faunal sampling associated to mussels, sulfide (ƩS) and iron (Fe(II)) concentrations were measured in situ using the chemical analyzer CHEMINI80 over the targeted mussel bed. Biological samples were collected using Victor6000’s manipulator arm (three to four grabs per sample) and placed in isotherm boxes. After grabs, a suction sampler was used on each sampling area to collect all remaining fauna. Sampled area was estimated analyzing Victor6000’s videos with the software ImageJ. Once on board, samples were sieved through 250 µm (directly fixed in 96° ethanol) and 20 µm (fixed with 4% buffered formalin, then in 96° ethanol, not considered in this study) mesh-sizes. Individuals were sorted, identified to the lowest taxonomic level possible using stereo- and binocular microscopes (except for mussel specimens, which were barcoded to identify the species), and counted (only individuals with complete anterior regions). Meiofaunal organisms (e.g., copepods and nematodes) found in the macrofaunal samples were included in the analyses.
Genetic analyses
We used the mitochondrial cytochrome oxidase I (mtCOI) to identify mussel specimens. In short, we extracted 10–50 mg of mussel tissue to be digested until total digestion with proteinase K at 60 °C in 0.5 ml pK-CTAB lysis buffer (containing 2% CTAB (Cetiltrimetilamina), 1 M NaCl, 1% PVP (Polyvinylpirrolidina), 20 mM EDTA pH8, 100 mM Tris–HCl pH 8, 0.1 mg mL-1 proteinase K).
Then, we: (1) extracted genomic DNA using the phenol/chloroform protocol, (2) precipitated it with Isopropanol and washes with ethanol 70%, (3) re-suspended and stored the pellet at − 20 °C in molecular quality sterile water until amplifications, (4) obtained partial sequences of the mtCOI gene using the specific primers:
BathCOI-F 5′-GTGGTCTGGAATAATTGGAAC-3′, and
BathCOI-R 5′-ATAAAAAGATGTATTRAARTGACG-3′
following Olu-Le Roy et al.81, and (5) amplified the DNA as follows: an initial step of denaturation at 94 °C for two minutes, five cycles of 35 s/94 °C, 35 s/48 °C and 70 s/72 °C, thirty-five cycles of 35 s/94 °C, 35 s/52 °C, 70 s/72 °C, and an final elongation at 72 °C for 10 min. We perform PCR reactions into a 25-ml reaction volume (1X PCR buffer, 2,2 mM MgCl2, 0.5 mM of each dNTPs, 0.55 µM of each primer, 0.02 U of Taq polymerase (GoTaq Promega), and 20 ng genomic DNA). The PCR products were purified and sequenced using the 3730XL Sequencer by thermofisher (Macrogen Europe, The Netherlands) following the manufacturer’s protocol.
Biodiversity and community composition
We estimated species diversity by individual-based rarefaction of Hill numbers (D)82 of q orders 0, 1 and 2, respectively. D expresses diversity in effective species number, i.e., the number of equally-common species that would represent the observed diversity, thus overcoming the problems of expressing indexes in different scales and respecting the “replication principle”82–84. When q = 0, 0D is the species number giving equal weight to abundant and rare species82,83. When q = 1, 1D is the exponential of Shannon index85 weighting species proportionally to their abundances and providing the effective number of common species in the assemblage82,83. When q = 2, 2D is the Simpson index86, which gives more weight to the dominant species and provides the effective number of dominant species in the assemblage82,83. We compared Capelinhos rarefied diversities (0D, 1D and 2D) to those from Cypress, Y3, Eiffel Tower and Montségur (LS), Atos (Menez Gwen), Thermitière and France (Rainbow) edifices using raw data from Sarrazin et al.44. Prior to comparisons, we removed copepod, nematode and ostracod taxa since we identified them at higher taxonomic resolutions than in previous studies. We estimated rarefaction curves and 95% confidence intervals for 1050 individuals and then plotted them using the iNEXT function of the iNEXT package87 in R V.4.0.2 environment88.
We compared Capelinhos species composition to those of LS, Menez Gwen, Broken Spur, Rainbow, TAG, Snake Pit, Logatchev and Ashadze-1 nMAR vent fields to disentangle biogeographic affinities based on the presence/absence dataset (including meiofauna) provided by Boschen-Rose and Colaço41. For consistency, we removed all taxa not identified at the species level. Moreover, we updated this dataset by including the annelid Ophryotrocha fabriae Paxton & Morineaux, 2009 and the nematode Oncholaimus dyvae Zeppilli et al., 2019, since both species inhabit the LS vent field55. We then computed a β-diversity dissimilarity distance matrix using Simpson’s pairwise dissimilarity metric (βsim), which ranges between 0 (no species compositional differences) to 1 (totally dissimilar species composition)89. βsim is independent of species richness differences and thus, only accounts for species turnover: it does not identify natural species-poor or unevenly-sampled communities as being highly dissimilar89,90. We computed βsim using the function beta.pair in the package betapart in R91. We performed a hierarchical cluster analysis (HCA) using the Average-linkage cluster algorithm, the βsim dissimilarity distance matrix and a multiscale bootstrap resampling to calculate the Approximately Unbiased p-values (AU-P) to identify robust clusters, with significant AU-P being set at > 9592. We estimated the bootstrap by repeatedly and randomly sampling sites performing the HCA by using the function pvclust in the package pvclust in R92.
Stable isotope analyses
We measured the carbon, nitrogen and sulfur isotopic ratios of 58 samples from 20 species/morphospecies (Appendix: Table S1), either using muscle tissue fragments (for mussels and shrimps), whole specimens or pools of specimens (Appendix: Table S1). For large species/individuals, we manually removed shells to avoid inorganic carbon bias. For smaller organisms with shells (e.g., the gastropods Protolira valvatoides Warén & Bouchet, 1993 and L. atlanticus Warén & Bouchet, 2001), we placed individuals in tin cups and acidified by direct addition of hydrochloric acid (HCl 1 M) 50 µl increments until detecting no bubbling93. We analyzed the isotopes at the University of Liege (Belgium) using a vario MICRO cube (Elementar, Germany) elemental analyzer coupled to an IsoPrime100 (Elementar, United Kingdom) isotope ratio mass spectrometer. We expressed isotope ratios using the δ notation94, in ‰ and relative to the international references: Vienna Pee Dee Belemnite (carbon), Atmospheric Air (nitrogen) and Vienna Canyon Diablo Troilite (sulfur). We used as primary analytical standards the following International Atomic Energy Agency (IAEA, Vienna, Austria) certified reference materials: sucrose (IAEA-C-6; δ13C = − 10.8 ± 0.5‰; mean ± SD), ammonium sulphate (IAEA-N-1; δ15N = 0.4 ± 0.2‰; mean ± SD), and silver sulfide (IAEA-S-1 δ34S = − 0.3‰). We used Sulfanilic acid (Sigma-Aldrich; δ13C = − 25.6 ± 0.4‰; δ15N = − 0.13 ± 0.4‰; δ34S = 5.9 ± 0.5‰; means ± SD) as a secondary analytical standard. Standard deviations on multi-batch replicate measurements of secondary and internal lab standards (seabass muscle) interspersed with samples (one replicate of each standard every 15 analyses) were 0.2‰ for both δ13C and δ15N and 0.4‰ for δ34S.
We used isotopic ratios to discuss the potential energy acquisition pathways of fauna and to compare the trophic structure with the close, well-characterized Eiffel Tower edifice at the main LS. At LS, δ13C values reflect methane oxidation, i.e., methanotrophy (− 12.9 ± 3.4‰) and/or sulfide oxidation, i.e., thiotrophy (− 36 to − 30‰, when using Calvin-Benson-Bassham cycle; − 15 to − 10‰, when using the reductive tricarboxylic acid cycle)65. Nitrogen reflects the use of nitrates (δ15N = 5–7‰) and/or ammonium (δ15N < 0‰)95,96. Particularly at LS, photosynthesis-derived organic matter range from − 24 to − 22‰ δ13C and from 4 to 6‰ δ15N69. δ34S values around or below 10‰ discern organic matter of chemosynthetic origin and, around or over 16‰, of photosynthetic origin72. We obtained the isotope values of Eiffel Tower assemblages from Alfaro-Lucas et al.56.
Supplementary Information
Acknowledgements
We thank the captains and crews of R/V Pourquoi pas? and the pilots of the ROV Victor6000 for their assistance at sea. Marjolaine Matabos, Bérengère Husson, Florence Pradillon, Emmanuelle Omnes, Nicolas Gayet et Philippe Rodier from the Biology and Ecology of Deep Sea Ecosystems Laboratory (Ifremer) are acknowledged for their invaluable assistance at sea and in the lab. JMAL warmly thanks the Deep-Sea Biology Society, which supported him with the Dive Deeper Research Bursary, and especially Dr Rachel Jeffreys, for the patience and attention during those difficult COVID times. This research program was funded by an ANR research grant (ANR Lucky Scales ANR-14-CE02-0008-02). The project is part of the EMSO-Azores regional node, and of EMSO France/EMSO ERIC Research Infrastructure. This paper is a contribution of DM to the Consolidated Research Group on Marine Benthic Ecology of the Generalitat de Catalunya (2021SGR00405).
Author contributions
J.S. collected the biological samples in the field and processed them on board. J.M.A.L. and D.M. sorted the samples in the laboratory and identified species. J.M.A.L. run the statistical analyses and wrote the original draft. L.N.M. run the stable isotope analyses. A.L. and C.C. obtained and analyzed chemical data. S.F. run the genetic analyses. All authors were involved in writing—review & editing of the final draft. J.S., J.M.A.L. and D.M. were involved the project administration and funding acquisition.
Data availability
Data are publicly available from the SEANOE repository (DOI: 10.17882/90421).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-52186-1.
References
- 1.Vellend M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010;85:183–206. doi: 10.1086/652373. [DOI] [PubMed] [Google Scholar]
- 2.HilleRisLambers J, Adler PB, Harpole WS, Levine JM, Mayfield MM. Rethinking community assembly through the lens of coexistence theory. Ecol. Evol. Syst. 2012;43:227–248. doi: 10.1146/annurev-ecolsys-110411-160411. [DOI] [Google Scholar]
- 3.Mittelbach GG, Schemske DW. Ecological and evolutionary perspectives on community assembly. Trends Ecol. Evol. 2015;30:241–247. doi: 10.1016/j.tree.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Leibold MA, Chase JM. Metacommunity ecology. In: Levin SA, Horn HS, editors. Monographs in Population Biology. Princeton University Press; 2018. p. 491. [Google Scholar]
- 5.Chase JM, Jeliazkov A, Ladouceur E, Viana DS. Biodiversity conservation through the lens of metacommunity ecology. Ann. N. Y. Acad. Sci. 2020;1469:86–104. doi: 10.1111/nyas.14378. [DOI] [PubMed] [Google Scholar]
- 6.Corliss JB, et al. Submarine thermal sprirngs on the galápagos rift. Science. 1979;203:1073–1083. doi: 10.1126/science.203.4385.1073. [DOI] [PubMed] [Google Scholar]
- 7.Jannasch HW, Mottl MJ. Geomicrobiology of deep-sea hydrothermal vents. Science. 1985;229:717–725. doi: 10.1126/science.229.4715.717. [DOI] [PubMed] [Google Scholar]
- 8.Johnson KS, Childress JJ, Beehler CL, Sakamoto CM. Biogeochemistry of hydrothermal vent mussel communities: The deep-sea analogue to the intertidal zone. Deep Sea Res. Part I Oceanogr. Res. Pap. 1994;41:993–1011. doi: 10.1016/0967-0637(94)90015-9. [DOI] [Google Scholar]
- 9.Govenar B. Shaping vent and seep communities: Habitat provision and modification by foundation species. In: Kiel S, editor. The Vent and Seep Biota Aspects from Microbes to Ecosystems. Springer; 2010. pp. 403–432. [Google Scholar]
- 10.Van Dover CL, German C, Speer K, Parson L, Vrijenhoek R. Evolution and biogeography of deep-sea vent and seep invertebrates. Science. 2002;295:1253–1257. doi: 10.1126/science.1067361. [DOI] [PubMed] [Google Scholar]
- 11.Mullineaux LS, et al. Exploring the ecology of deep-sea hydrothermal vents in a metacommunity framework. Front. Mar. Sci. 2018;5:49. doi: 10.3389/fmars.2018.00049. [DOI] [Google Scholar]
- 12.Bris N, et al. Hydrothermal energy transfer and organic carbon production at the deep seafloor. Front. Mar. Sci. 2019;5:531. doi: 10.3389/fmars.2018.00531. [DOI] [Google Scholar]
- 13.Beaulieu SE, Baker ET, German CR. Where are the undiscovered hydrothermal vents on oceanic spreading ridges? Deep Sea Res. Part II Top. Stud. Oceanogr. 2015;121:202–212. doi: 10.1016/j.dsr2.2015.05.001. [DOI] [Google Scholar]
- 14.Van Dover CL, et al. Scientific rationale and international obligations for protection of active hydrothermal vent ecosystems from deep-sea mining. Mar. Policy. 2018;90:20–28. doi: 10.1016/j.marpol.2018.01.020. [DOI] [Google Scholar]
- 15.Levin LA, Le Bris N. The deep ocean under climate change. Science. 2015;350:766–768. doi: 10.1126/science.aad0126. [DOI] [PubMed] [Google Scholar]
- 16.Van Dover CL. Inactive sulfide ecosystems in the deep sea: A review. Front. Mar. Sci. 2019;6:461. doi: 10.3389/fmars.2019.00461. [DOI] [Google Scholar]
- 17.Gollner S, et al. Application of scientific criteria for identifying hydrothermal ecosystems in need of protection. Mar. Policy. 2021;132:104641. doi: 10.1016/j.marpol.2021.104641. [DOI] [Google Scholar]
- 18.Tunnicliffe V, Fowler CMR. Influence of sea-floor spreading on the global hydrothermal vent fauna. Nature. 1996;379:531–533. doi: 10.1038/379531a0. [DOI] [Google Scholar]
- 19.Moalic Y, et al. Biogeography revisited with network theory: Retracing the history of hydrothermal vent communities. Syst. Biol. 2012;61:127–137. doi: 10.1093/sysbio/syr088. [DOI] [PubMed] [Google Scholar]
- 20.Rogers AD, et al. The discovery of new deep-sea hydrothermal vent communities in the southern ocean and implications for biogeography. PLoS Biol. 2012;10:e1001234. doi: 10.1371/journal.pbio.1001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh AG, Mullineaux LS, Young CM, Manahan DT. Larval dispersal potential of the tubeworm Riftia pachyptila at deep-sea hydrothermal vents. Nature. 2001;411:77–80. doi: 10.1038/35075063. [DOI] [PubMed] [Google Scholar]
- 22.Breusing C, et al. Biophysical and population genetic models predict the presence of “Phantom” stepping stones connecting mid-atlantic ridge vent ecosystems. Curr. Biol. 2016;26:2257–2267. doi: 10.1016/j.cub.2016.06.062. [DOI] [PubMed] [Google Scholar]
- 23.Vrijenhoek RC. Genetic diversity and connectivity of deep-sea hydrothermal vent metapopulations. Mol. Ecol. 2010;19:4391–4411. doi: 10.1111/j.1365-294X.2010.04789.x. [DOI] [PubMed] [Google Scholar]
- 24.Mitarai S, Watanabe H, Nakajima Y, Shchepetkin AF, McWilliams JC. Quantifying dispersal from hydrothermal vent fields in the western Pacific Ocean. Proc. Natl. Acad. Sci. 2016;113:2976–2981. doi: 10.1073/pnas.1518395113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vic C, Gula J, Roullet G, Pradillon F. Dispersion of deep-sea hydrothermal vent effluents and larvae by submesoscale and tidal currents. Deep Sea Res. Part Oceanogr. Res. Pap. 2018;133:1–18. doi: 10.1016/j.dsr.2018.01.001. [DOI] [Google Scholar]
- 26.Yearsley JM, Salmanidou DM, Carlsson J, Burns D, Dover CLV. Biophysical models of persistent connectivity and barriers on the northern Mid-Atlantic Ridge. Deep Sea Res. Part II Top. Stud. Oceanogr. 2020;180:104819. doi: 10.1016/j.dsr2.2020.104819. [DOI] [Google Scholar]
- 27.Desbruyères D, et al. A review of the distribution of hydrothermal vent communities along the northern Mid-Atlantic Ridge: Dispersal vs. environmental controls. Hydrobiologia. 2000;440:201–216. doi: 10.1023/A:1004175211848. [DOI] [Google Scholar]
- 28.Desbruyères D, et al. Variations in deep-sea hydrothermal vent communities on the Mid-Atlantic Ridge near the Azores plateau. Deep Sea Res. Part I Oceanogr. Res. Pap. 2001;48:1325–1346. doi: 10.1016/S0967-0637(00)00083-2. [DOI] [Google Scholar]
- 29.Goffredi SK, et al. Hydrothermal vent fields discovered in the southern Gulf of California clarify role of habitat in augmenting regional diversity. Proc. R. Soc. B. 2017;284:20170817. doi: 10.1098/rspb.2017.0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giguère TN, Tunnicliffe V. Beta diversity differs among hydrothermal vent systems: Implications for conservation. PLoS ONE. 2021;16:e0256637. doi: 10.1371/journal.pone.0256637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarrazin J, Robigou V, Juniper K, Delaney J. Biological and geological dynamics over four years on a high-temperature sulfide structure at the Juan de Fuca Ridge hydrothermal observatory. Meps. 1997;153:5–24. doi: 10.3354/meps153005. [DOI] [Google Scholar]
- 32.Kelly N, Metaxas A. Diversity of invertebrate colonists on simple and complex substrates at hydrothermal vents on the Juan de Fuca Ridge. Aquat. Biol. 2008;3:271–281. doi: 10.3354/ab00085. [DOI] [Google Scholar]
- 33.Micheli F, et al. Predation structures communities at deep-sea hydrothermal vents. Ecol. Monogr. 2002;72:365–382. doi: 10.1890/0012-9615(2002)072[0365:PSCADS]2.0.CO;2. [DOI] [Google Scholar]
- 34.Mullineaux LS, Peterson CH, Micheli F, Mills SW. Successional mechanism varies along a gradient in hydrothermal fluid flux at deep-sea vents. Ecol. Monogr. 2003;73:523–542. doi: 10.1890/02-0674. [DOI] [Google Scholar]
- 35.Mullineaux LS, et al. Detecting the influence of initial pioneers on succession at deep-sea vents. PLoS ONE. 2012;7:e50015. doi: 10.1371/journal.pone.0050015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mullineaux LS, Adams DK, Mills SW, Beaulieu SE. Larvae from afar colonize deep-sea hydrothermal vents after a catastrophic eruption. Proc. Natl. Acad. Sci. 2010;107:7829–7834. doi: 10.1073/pnas.0913187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brunner O, et al. Species assemblage networks identify regional connectivity pathways among hydrothermal vents in the Northwest Pacific. Ecol. Evol. 2022;12:e9612. doi: 10.1002/ece3.9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, et al. Delineating biogeographic regions in Indian Ocean deep-sea vents and implications for conservation. Divers. Distrib. 2022 doi: 10.1111/ddi.13535. [DOI] [Google Scholar]
- 39.Van Dover CL. Ecology of Mid-Atlantic Ridge hydrothermal vents. Geol. Soc. Lond. Spec. Publ. 1995;87:257–294. doi: 10.1144/GSL.SP.1995.087.01.21. [DOI] [Google Scholar]
- 40.Goroslavskaya E, Galkin S. Hydrothermal assemblages associated with different foundation species on the East Pacific Rise and Mid-Atlantic Ridge, with a special focus on mytilids. Mar. Ecol. 2015;36:45–61. doi: 10.1111/maec.12262. [DOI] [Google Scholar]
- 41.Boschen-Rose RE, Colaço A. Northern Mid-Atlantic Ridge hydrothermal habitats: A systematic review of knowledge status for environmental management. Front. Mar. Sci. 2021;8:657358. doi: 10.3389/fmars.2021.657358. [DOI] [Google Scholar]
- 42.Wheeler AJ, et al. Moytirra: Discovery of the first known deep-sea hydrothermal vent field on the slow-spreading Mid-Atlantic Ridge north of the Azores. Geochem. Geophys. Geosyst. 2013;14:4170–4184. doi: 10.1002/ggge.20243. [DOI] [Google Scholar]
- 43.Sarrazin J, et al. Integrated study of new faunal assemblages dominated by gastropods at three vent fields along the Mid-Atlantic Ridge: Diversity, structure, composition and trophic interactions. Front. Mar. Sci. 2022;9:925419. doi: 10.3389/fmars.2022.925419. [DOI] [Google Scholar]
- 44.Sarrazin J, et al. Endogenous versus exogenous factors: What matters for vent mussel communities? Deep Sea Res. Part Oceanogr. Res. Pap. 2020;160:103260. doi: 10.1016/j.dsr.2020.103260. [DOI] [Google Scholar]
- 45.O’Mullan GD, Maas PAY, Lutz RA, Vrijenhoek RC. A hybrid zone between hydrothermal vent mussels (Bivalvia: Mytilidae) from the Mid-Atlantic Ridge. Mol. Ecol. 2001;10:2819–2831. doi: 10.1046/j.0962-1083.2001.01401.x. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Rey M, Serafim A, Company R, Bebianno MJ. Adaptation to metal toxicity: A comparison of hydrothermal vent and coastal shrimps. Mar. Ecol. 2007;28:100–107. doi: 10.1111/j.1439-0485.2006.00126.x. [DOI] [Google Scholar]
- 47.Fabri M-C, et al. The hydrothermal vent community of a new deep-sea field, Ashadze-1, 12° 58′ N on the Mid-Atlantic Ridge. J. Mar. Biol. Assoc. UK. 2011;91:1–13. doi: 10.1017/S0025315410000731. [DOI] [Google Scholar]
- 48.Kelley DS, et al. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30° N. Nature. 2001;412:145–149. doi: 10.1038/35084000. [DOI] [PubMed] [Google Scholar]
- 49.DeChaine EG, Bates AE, Shank TM, Cavanaugh CM. Off-axis symbiosis found: Characterization and biogeography of bacterial symbionts of Bathymodiolus mussels from Lost City hydrothermal vents. Environ. Microbiol. 2006;8:1902–1912. doi: 10.1111/j.1462-2920.2005.01113.x. [DOI] [PubMed] [Google Scholar]
- 50.Lartaud F, et al. Fossil evidence for serpentinization fluids fueling chemosynthetic assemblages. Proc. Natl. Acad. Sci. USA. 2011;108:7698–7703. doi: 10.1073/pnas.1009383108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryan WBF, et al. Global multi-resolution topography synthesis. Geochem. Geophys. Geosyst. 2009 doi: 10.1029/2008GC002332. [DOI] [Google Scholar]
- 52.Escartin J, et al. Hydrothermal activity along the slow-spreading Lucky Strike ridge segment (Mid-Atlantic Ridge): Distribution, heatflux, and geological controls. Earth Planet. Sci. Lett. 2015;431:173–185. doi: 10.1016/j.epsl.2015.09.025. [DOI] [Google Scholar]
- 53.Chavagnac V, et al. Spatial variations in vent chemistry at the lucky strike hydrothermal field, Mid-Atlantic Ridge (37°N): Updates for subseafloor flow geometry from the newly discovered capelinhos vent. Geochem. Geophys. Geosyst. 2018;19:4444–4458. doi: 10.1029/2018GC007765. [DOI] [Google Scholar]
- 54.Marticorena J, et al. Recovery of hydrothermal vent communities in response to an induced disturbance at the Lucky Strike vent field (Mid-Atlantic Ridge) Mar. Environ. Res. 2021;168:105316. doi: 10.1016/j.marenvres.2021.105316. [DOI] [PubMed] [Google Scholar]
- 55.Alfaro-Lucas JM, et al. High environmental stress and productivity increase functional diversity along a deep-sea hydrothermal vent gradient. Ecology. 2020 doi: 10.1002/ecy.3144. [DOI] [PubMed] [Google Scholar]
- 56.Alfaro-Lucas JM, 2019. Abundance, functional traits and stable isotopes of species colonizing slate and wood substrata along a vent gradient at and away from the Eiffel Tower edifice (Lucky Strike vent field, Mid-Atlantic Ridge) SEANOE. [DOI]
- 57.Cuvelier D, et al. Community dynamics over 14 years at the Eiffel Tower hydrothermal edifice on the Mid-Atlantic Ridge. Limnol. Oceanogr. 2011;56:1624–1640. doi: 10.4319/lo.2011.56.5.1624. [DOI] [Google Scholar]
- 58.Cuvelier D, Sarrazin J, Colaço A, Copley J, Desbruyères D, Glover AG, Tyler P, Santos RS. Distribution and spatial variation of hydrothermal faunal assemblages at Lucky Strike (Mid-Atlantic Ridge) revealed by high-resolution video image analysis. Deep Sea Res. Part I Oceanogr. Res. Pap. 2009;56(11):2026. doi: 10.1016/j.dsr.2009.06.006. [DOI] [Google Scholar]
- 59.Sarrazin J, et al. Biodiversity patterns, environmental drivers and indicator species on a high-temperature hydrothermal edifice, Mid-Atlantic Ridge. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015;121:177–192. doi: 10.1016/j.dsr2.2015.04.013. [DOI] [Google Scholar]
- 60.Husson B, Sarradin P-M, Zeppilli D, Sarrazin J. Picturing thermal niches and biomass of hydrothermal vent species. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017;137:6–25. doi: 10.1016/j.dsr2.2016.05.028. [DOI] [Google Scholar]
- 61.Van Dover CL, et al. Biology of the Lucky Strike hydrothermal field. Deep Sea Res. Part I Oceanogr. Res. Pap. 1996;43:1509–1529. doi: 10.1016/S0967-0637(96)00051-9. [DOI] [Google Scholar]
- 62.Van Dover CL. Variation in community structure within hydrothermal vent mussel beds of the East Pacific Rise. Mar. Ecol. Prog. Ser. 2003 doi: 10.3354/meps253055. [DOI] [Google Scholar]
- 63.Govenar B, et al. Epifaunal community structure associated with Riftia pachyptila aggregations in chemically different hydrothermal vent habitats. Mar. Ecol. Prog. Ser. 2005;305:67–77. doi: 10.3354/meps305067. [DOI] [Google Scholar]
- 64.Busserolles FD, et al. Are spatial variations in the diets of hydrothermal fauna linked to local environmental conditions? Deep Sea Res. Part II Top. Stud. Oceanogr. 2009;56:1649–1664. doi: 10.1016/j.dsr2.2009.05.011. [DOI] [Google Scholar]
- 65.Portail M, et al. Food-web complexity across hydrothermal vents on the Azores triple junction. Deep Sea Res. Part I Oceanogr. Res. Pap. 2018;131:101–120. doi: 10.1016/j.dsr.2017.11.010. [DOI] [Google Scholar]
- 66.Waeles M, et al. On the early fate of hydrothermal iron at deep-sea vents: A reassessment after in situ filtration. Geophys. Res. Lett. 2017;44:4233–4240. doi: 10.1002/2017GL073315. [DOI] [Google Scholar]
- 67.Cotte L, et al. Metal partitioning after in situ filtration at deep-sea vents of the Lucky Strike hydrothermal field (EMSO-Azores, Mid-Atlantic Ridge, 37° N) Deep Sea Res. Part Oceanogr. Res. Pap. 2020;157:103204. doi: 10.1016/j.dsr.2019.103204. [DOI] [Google Scholar]
- 68.Govenar B, Fisher CR. Experimental evidence of habitat provision by aggregations of Riftia pachyptila at hydrothermal vents on the East Pacific Rise. Mar. Ecol. 2007;28:3–14. doi: 10.1111/j.1439-0485.2007.00148.x. [DOI] [Google Scholar]
- 69.Charlou JL, et al. High production and fluxes of H2 and CH4 and evidence of abiotic hydrocarbon synthesis by serpentinization in ultramafic-hosted hydrothermal systems on the Mid-Atlantic Ridge. In: Rona PA, Devey CW, Dyment J, Murton BJ, et al., editors. Geophysical Monograph Series. American Geophysical Union; 2010. pp. 265–296. [Google Scholar]
- 70.Colaço A, Dehairs F, Desbruyères D. Nutritional relations of deep-sea hydrothermal fields at the Mid-Atlantic Ridge: A stable isotope approach. Deep Sea Res. Part I Oceanogr. Res. Pap. 2002;49:395–412. doi: 10.1016/S0967-0637(01)00060-7. [DOI] [Google Scholar]
- 71.Trask JL, Van Dover CL. Site-specific and ontogenetic variations in nutrition of mussels (Bathymodiolus sp.) from the Lucky Strike hydrothermal vent field, Mid-Atlantic Ridge. Limnol. Oceanogr. 1999;44:334–343. doi: 10.4319/lo.1999.44.2.0334. [DOI] [Google Scholar]
- 72.Reid WD, et al. Spatial differences in East Scotia Ridge hydrothermal vent food webs: Influences of chemistry, microbiology and predation on trophodynamics. PLoS ONE. 2013;8:e65553. doi: 10.1371/journal.pone.0065553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vetter RD, Fry B. Sulfur contents and sulfur-isotope compositions of thiotrophic symbioses in bivalve molluscs and vestimentiferan worms. Mar. Biol. 1998;132:453–460. doi: 10.1007/s002270050411. [DOI] [Google Scholar]
- 74.Sánchez-Mora D, Jamieson J, Cannat M, Escartín J, Barreyre T. Effects of substrate composition and subsurface fluid pathways on the geochemistry of seafloor hydrothermal deposits at the lucky strike vent field, Mid-Atlantic Ridge. Geochem. Geophys. Geosyst. 2022;23:e2021GC010073. doi: 10.1029/2021GC010073. [DOI] [Google Scholar]
- 75.Riou V, et al. Influence of CH4 and H2S availability on symbiont distribution, carbon assimilation and transfer in the dual symbiotic vent mussel Bathymodiolus azoricus. Biogeosciences. 2008;5:1681–1691. doi: 10.5194/bg-5-1681-2008. [DOI] [Google Scholar]
- 76.Duperron S, et al. A dual symbiosis shared by two mussel species, Bathymodiolus azoricus and Bathymodiolus puteoserpentis (Bivalvia: Mytilidae), from hydrothermal vents along the northern Mid-Atlantic Ridge. Environ. Microbiol. 2006;8:1441–1447. doi: 10.1111/j.1462-2920.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 77.Somoza L, et al. Multidisciplinary scientific cruise to the northern Mid-Atlantic Ridge and Azores Archipelago. Front. Mar. Sci. 2020;7:568035. doi: 10.3389/fmars.2020.568035. [DOI] [Google Scholar]
- 78.Langmuir C, et al. Hydrothermal vents near a mantle hot spot: The Lucky Strike vent field at 37° N on the Mid-Atlantic Ridge. Earth Planet. Sci. Lett. 1997;148:69–91. doi: 10.1016/S0012-821X(97)00027-7. [DOI] [Google Scholar]
- 79.Ondréas H, et al. Recent volcanic events and the distribution of hydrothermal venting at the Lucky Strike hydrothermal field, Mid-Atlantic Ridge. Geochem. Geophys. Geosyst. 2009 doi: 10.1029/2008GC002171. [DOI] [Google Scholar]
- 80.Vuillemin R, et al. CHEMINI: A new in situ CHEmical MINIaturized analyzer. Deep Sea Res. Part Oceanogr. Res. Pap. 2009;56:1391–1399. doi: 10.1016/j.dsr.2009.02.002. [DOI] [Google Scholar]
- 81.Roy KO-L, von Cosel R, Hourdez S, Carney SL, Jollivet D. Amphi-Atlantic cold-seep Bathymodiolus species complexes across the equatorial belt. Deep Sea Res. Part Oceanogr. Res. Pap. 2007;54:1890–1911. doi: 10.1016/j.dsr.2007.07.004. [DOI] [Google Scholar]
- 82.Chao A, et al. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014;84:45–67. doi: 10.1890/13-0133.1. [DOI] [Google Scholar]
- 83.Jost L. Entropy and diversity. Oikos. 2006;113:363–375. doi: 10.1111/j.2006.0030-1299.14714.x. [DOI] [Google Scholar]
- 84.Roswell M, Dushoff J, Winfree R. A conceptual guide to measuring species diversity. Oikos. 2021 doi: 10.1111/oik.07202. [DOI] [Google Scholar]
- 85.Shannon CE. A mathematical theory of communication. Bell Syst. Tech. J. 1948;27:379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x. [DOI] [Google Scholar]
- 86.Simpson EH. Measurement of diversity. Nature. 1949;163:688–688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- 87.Hsieh TC, Ma KH, Chao A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers) Methods Ecol. Evol. 2016;7:1451–1456. doi: 10.1111/2041-210X.12613. [DOI] [Google Scholar]
- 88.Team RCR . The R Project for Statistical Computing. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 89.Baselga A. Partitioning the turnover and nestedness components of beta diversity. Glob. Ecol. Biogeogr. 2010;19:134–143. doi: 10.1111/j.1466-8238.2009.00490.x. [DOI] [Google Scholar]
- 90.Kreft H, Jetz W. A framework for delineating biogeographical regions based on species distributions. J. Biogeogr. 2010;37:2029–2053. doi: 10.1111/j.1365-2699.2010.02375.x. [DOI] [Google Scholar]
- 91.Baselga A, Orme DC. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012;3:808–812. doi: 10.1111/j.2041-210X.2012.00224.x. [DOI] [Google Scholar]
- 92.Suzuki R, Shimodaira H. Pvclust: An R package for assessing the uncertainty in hierarchical clustering. Bioinformatics. 2006;22:1540–1542. doi: 10.1093/bioinformatics/btl117. [DOI] [PubMed] [Google Scholar]
- 93.Jaschinski S, Hansen T, Sommer U. Effects of acidification in multiple stable isotope analyses. Limnol. Oceanogr. Methods. 2008;6:12–15. doi: 10.4319/lom.2008.6.12. [DOI] [Google Scholar]
- 94.Coplen TB. Isotope reference materials. In: Beauchemin D, Matthews D, editors. The Encyclopedia of Mass Spectrometry. Elsevier; 2010. pp. 774–783. [Google Scholar]
- 95.Lee R, Childress J. Inorganic N assimilation and ammonium pools in a deep-sea mussel containing methanotrophic endosymbionts. Biol. Bull. 1996;190:373–384. doi: 10.2307/1543030. [DOI] [PubMed] [Google Scholar]
- 96.Riekenberg P, Carney R, Fry B. Trophic plasticity of the methanotrophic mussel Bathymodiolus childressi in the Gulf of Mexico. Mar. Ecol. Prog. Ser. 2016;547:91–106. doi: 10.3354/meps11645. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Alfaro-Lucas JM, 2019. Abundance, functional traits and stable isotopes of species colonizing slate and wood substrata along a vent gradient at and away from the Eiffel Tower edifice (Lucky Strike vent field, Mid-Atlantic Ridge) SEANOE. [DOI]
Supplementary Materials
Data Availability Statement
Data are publicly available from the SEANOE repository (DOI: 10.17882/90421).