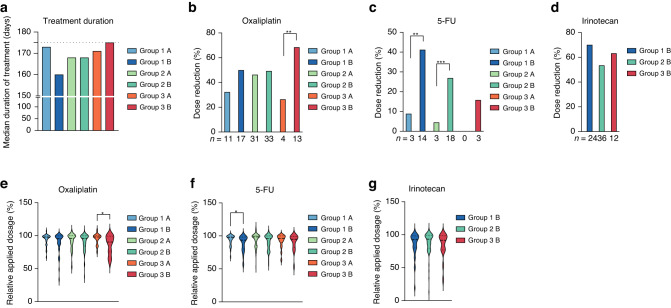
Fig. 2. Dose intensity and duration of treatment according to clinical groups.
a Time of treatment is shown as the median time of treatment in days per treatment arm. Dose reductions (-at least 5% of medication) during the induction phase of the study are shown as relative per treatment arm for oxaliplatin (b), 5-FU (c) or irinotecan (d). The relative applied dosage of oxaliplatin (e), 5-FU (f) or irinotecan (g) was calculated as relative to the maximal applied dosage per protocol and patient. *P < 0.05, **P < 0.001, ***P < 0.0001, determined by two-tailed chi-square test (b, c) or unpaired t test (e–g). Arm A is FOLFOX/bevacizumab and arm B FOLFOXIRI/bevacizumab.