Abstract
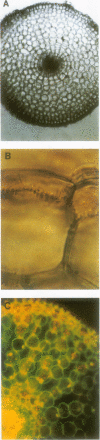
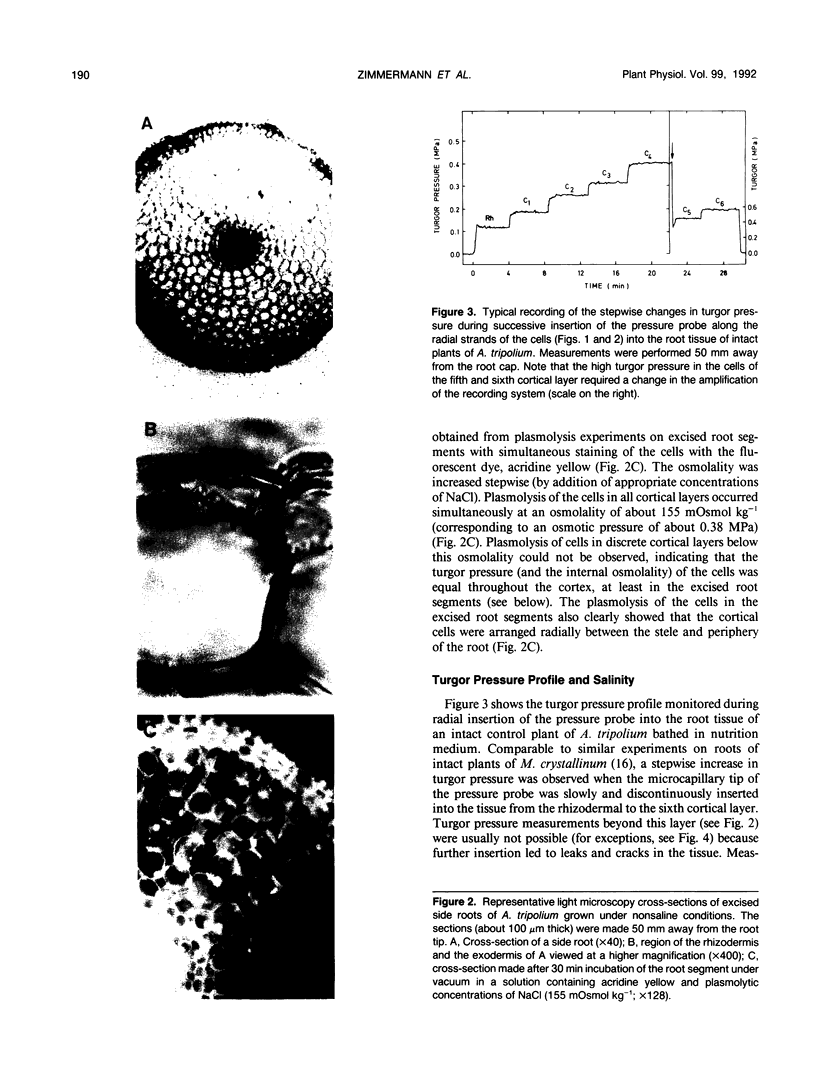
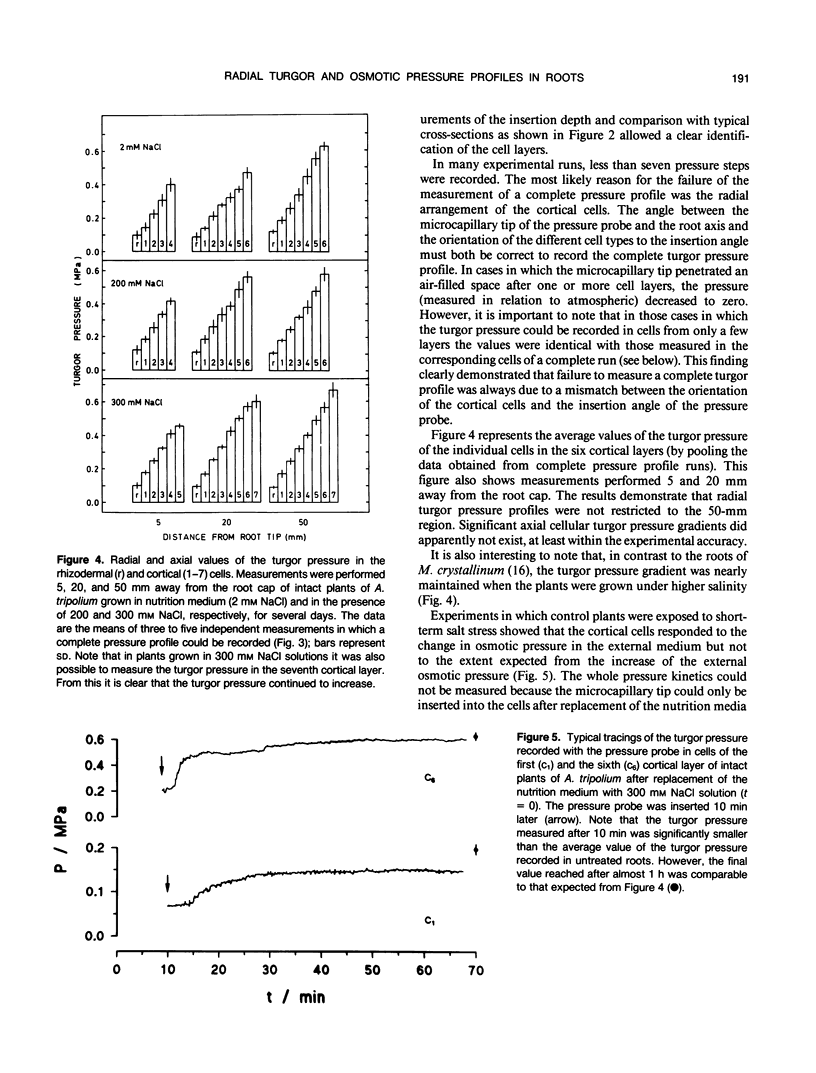
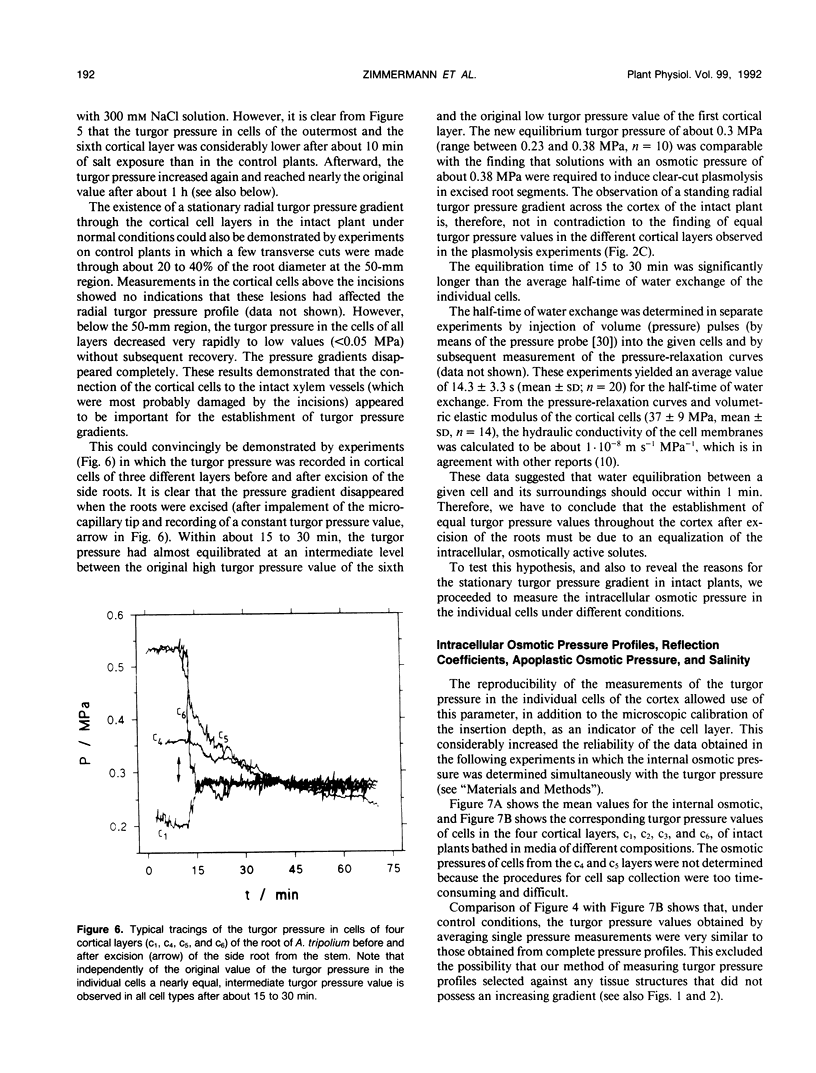
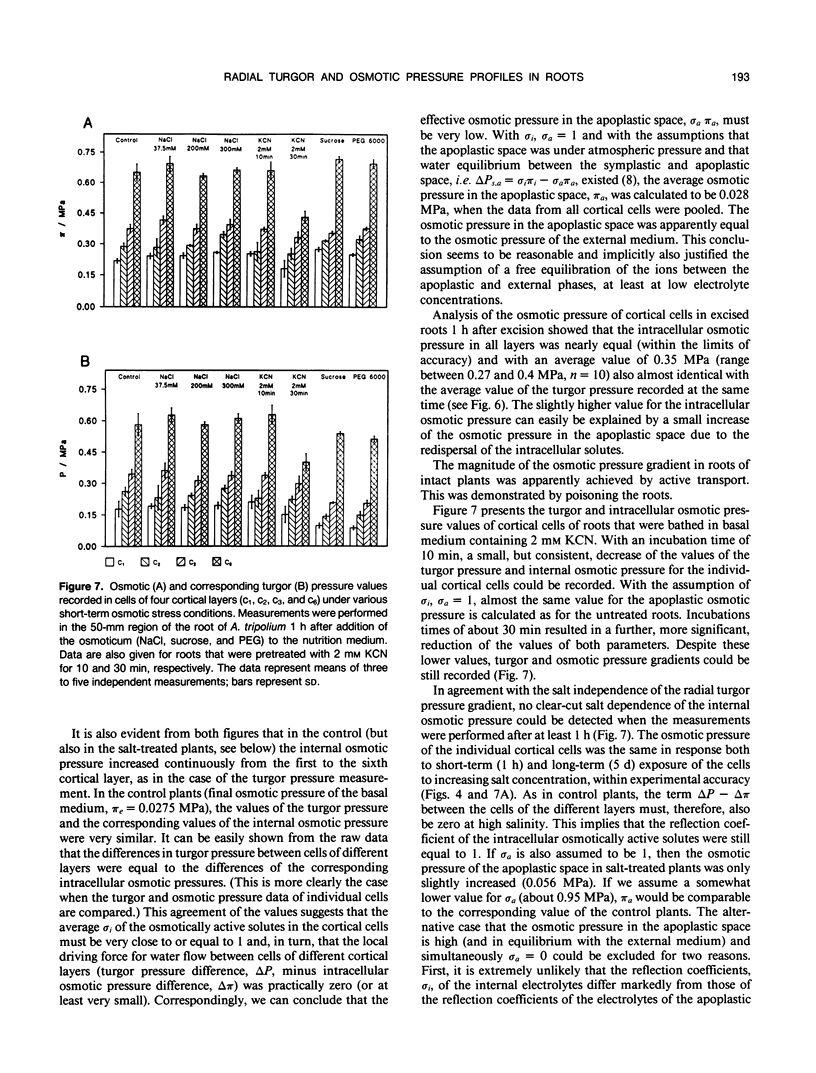
High-resolution nuclear magnetic resonance images (using very short spin-echo times of 3.8 milliseconds) of cross-sections of excised roots of the halophyte Aster tripolium showed radial cell strands separated by air-filled spaces. Radial insertion of the pressure probe (along the cell strands) into roots of intact plants revealed a marked increase of the turgor pressure from the outermost to the sixth cortical layer (from about 0.1-0.6 megapascals). Corresponding measurements of intracellular osmotic pressure in individual cortical cells (by means of a nanoliter osmometer) showed an osmotic pressure gradient of equal magnitude to the turgor pressure. Neither gradient changed significantly when the plants were grown in, or exposed for 1 hour to, media of high salinity. Differences were recorded in the ability of salts and nonelectrolytes to penetrate the apoplast in the root. The reflection coefficients of the cortical cells were approximately 1 for all the solutes tested. Excision of the root from the stem resulted in a collapse of the turgor and osmotic pressure gradients. After about 15 to 30 minutes, the turgor pressure throughout the cortex attained an intermediate (quasistationary) level of about 0.3 megapascals. This value agreed well with the osmotic value deduced from plasmolysis experiments on excised root segments. These and other data provided conclusions about the driving forces for water and solute transport in the roots and about the function of the air-filled radial spaces in water transport. They also showed that excised roots may be artifactual systems.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. M., Johnson G. A., Kramer P. J. In Vivo Magnetic Resonance Microscopy of Changing Water Content in Pelargonium hortorum Roots. Plant Physiol. 1986 Dec;82(4):1158–1160. doi: 10.1104/pp.82.4.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscus E. L. Determination of hydraulic and osmotic properties of soybean root systems. Plant Physiol. 1977 Jun;59(6):1013–1020. doi: 10.1104/pp.59.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiscus E. L. The Interaction between Osmotic- and Pressure-induced Water Flow in Plant Roots. Plant Physiol. 1975 May;55(5):917–922. doi: 10.1104/pp.55.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. M. Studies of Root Function in Zea mays: III. Xylem Sap Composition at Maximum Root Pressure Provides Evidence of Active Transport into the Xylem and a Measurement of the Reflection Coefficient of the Root. Plant Physiol. 1985 Jan;77(1):162–167. doi: 10.1104/pp.77.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E., Oren R., Schulze E. D. Water transport in maize roots : measurement of hydraulic conductivity, solute permeability, and of reflection coefficients of excised roots using the root pressure probe. Plant Physiol. 1987 Aug;84(4):1220–1232. doi: 10.1104/pp.84.4.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E., Zimmermann U. Hydraulische Leitfähigkeit von Valonia utricularis. Z Naturforsch B. 1971 Dec;26(12):1302–1311. [PubMed] [Google Scholar]