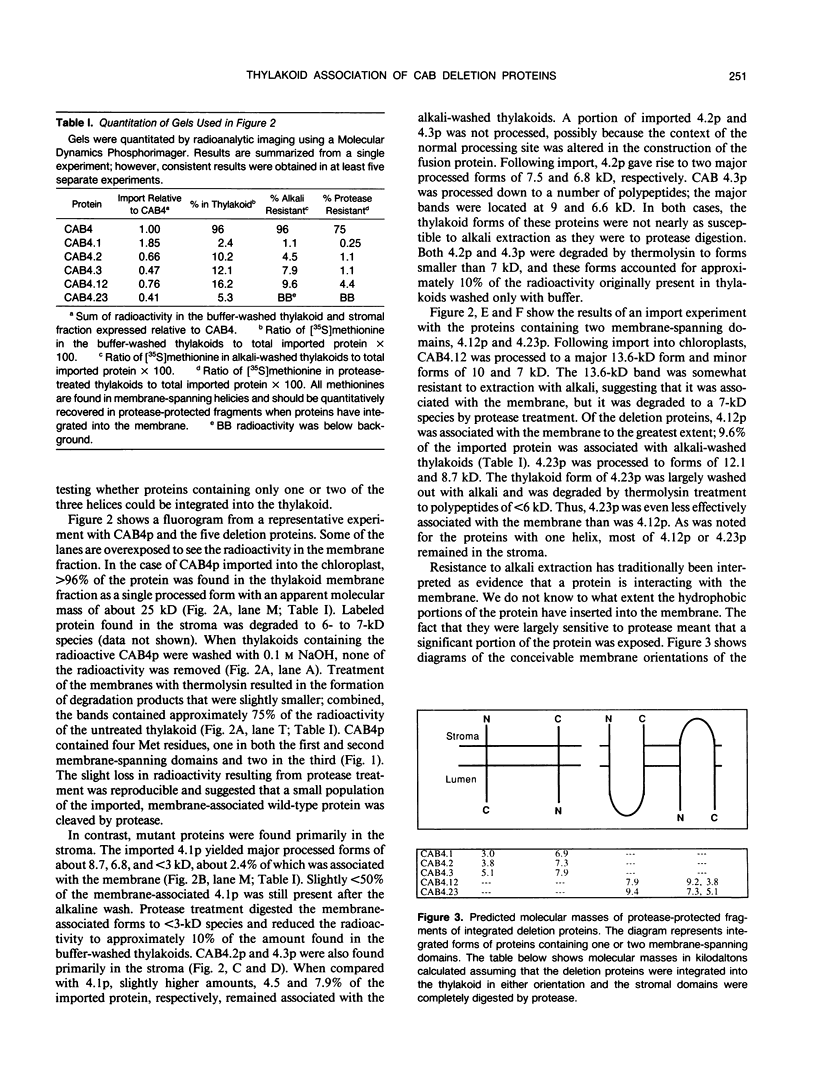
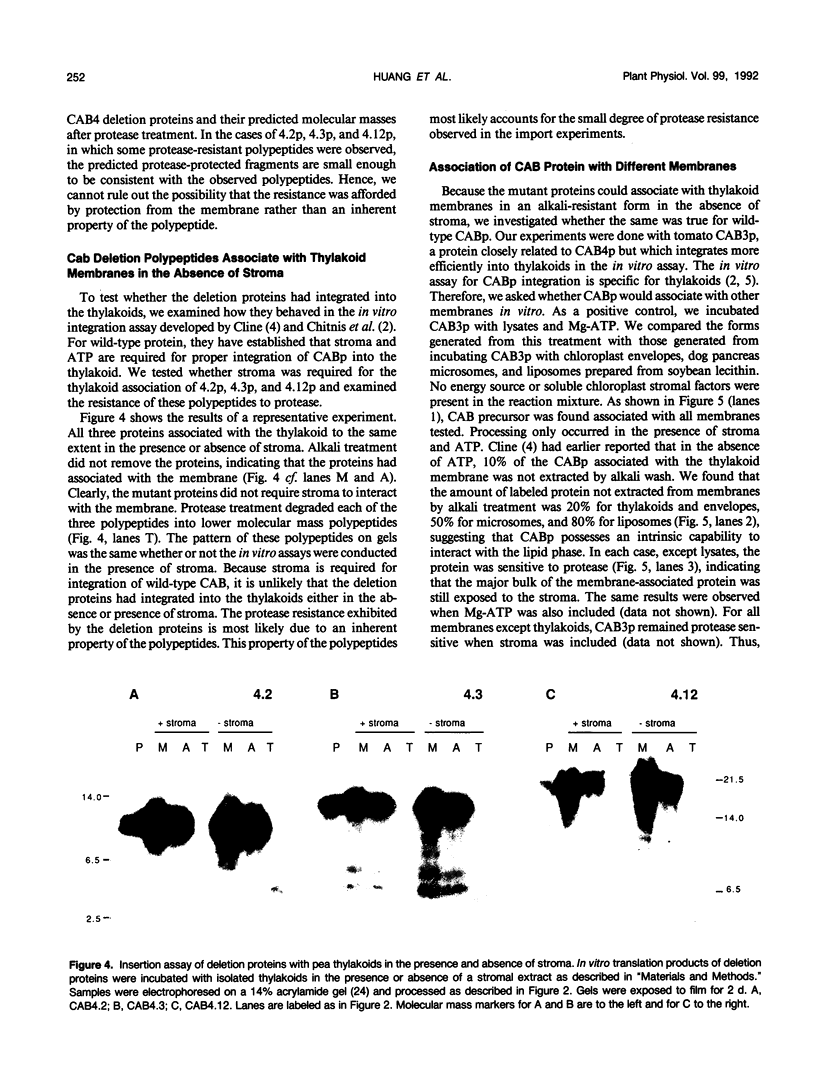
Abstract
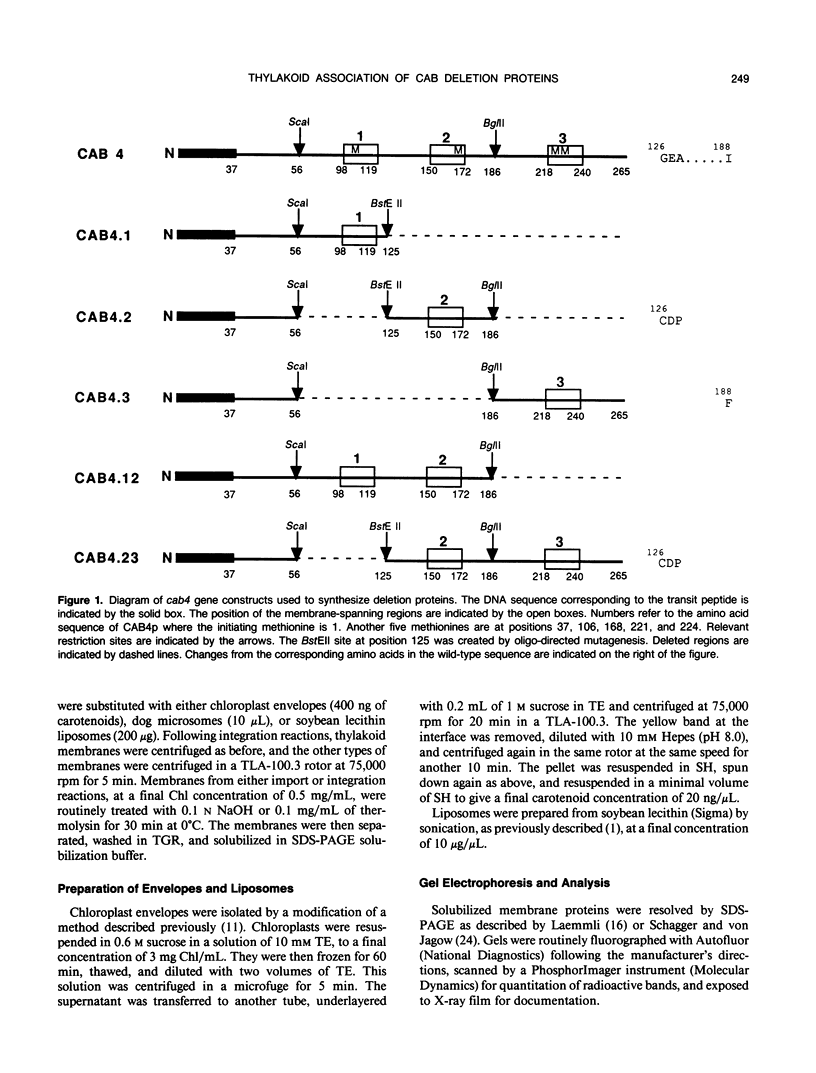
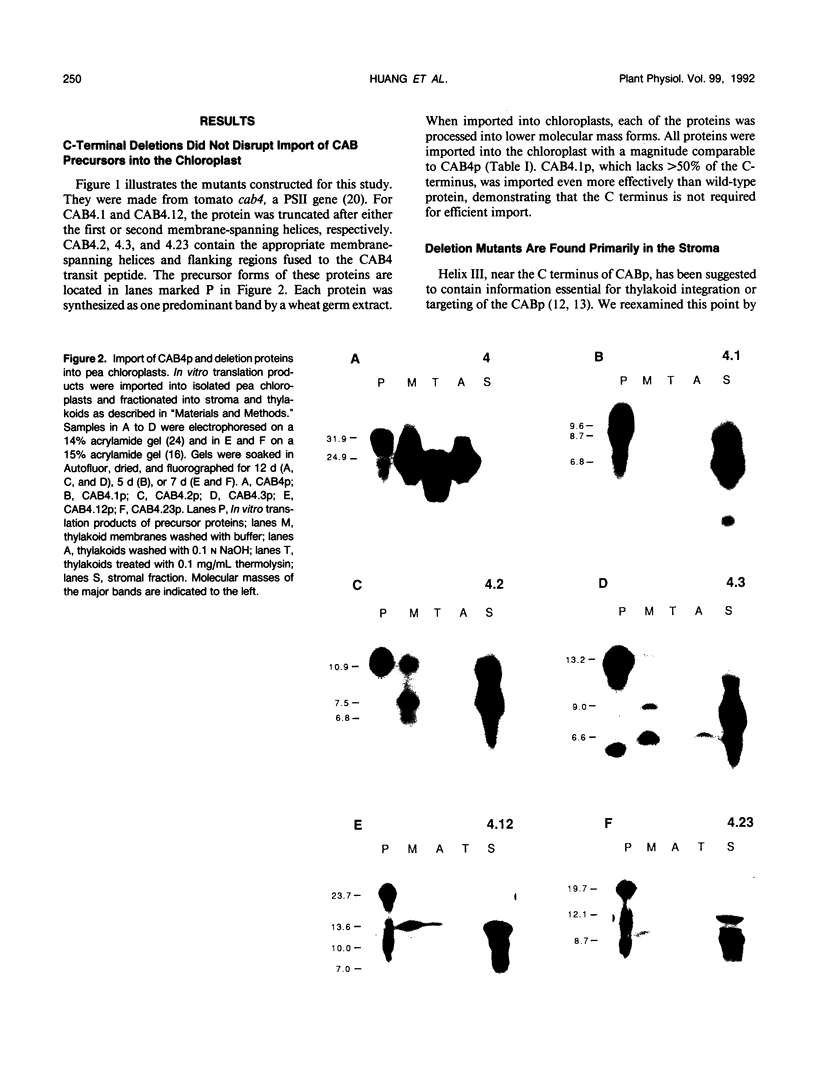
Chlorophyll a/b binding polypeptides (CABp) are integral thylakoid membrane proteins containing three membrane-spanning helices. We have created a series of mutations in tomato CABp to test whether individual membrane helices with hydrophilic flanking sequences, when fused to a transit peptide, can be imported into chloroplasts and correctly targeted to thylakoid membranes. All of the mutated precursors, including those with large C-terminal and internal deletions, were imported successfully, showing that these regions of the mature CABp are not required for import into chloroplasts. All mutants tested, containing either one or two membrane helices, were found primarily in the stroma and not in the thylakoids. The small amount of protein found associated with the thylakoids was largely resistant to alkali extraction but was sensitive to protease, unlike wild-type protein, which is resistant to both treatments. When incubated with thylakoids in the absence of stroma and/or ATP, a significant amount of wild-type protein assumes a form that is resistant to alkali extraction but is protease sensitive, like the imported deletion proteins. This form of the wild-type protein is not chased into a protease-resistant form by adding stroma and/or ATP. These results suggest that CABp can spontaneously associate with membranes as an aberrant species that is not an intermediate in the process of integration. The inability of the deletion forms of CABp to assume a protease-resistant conformation suggests that correct integration is afforded by elements within the entire protein that collectively contribute to the proper conformation of the protein. The ability of deletion mutants to associate with thylakoids in a nonphysiological way suggests that the study of such mutants may not be useful in elucidating thylakoid-targeting signals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam Z., Malkin R. Reconstitution of isolated Rieske Fe-S protein into a Rieske-depleted cytochrome b6-f complex. FEBS Lett. 1987 Dec 10;225(1-2):67–71. doi: 10.1016/0014-5793(87)81132-8. [DOI] [PubMed] [Google Scholar]
- Clark S. E., Oblong J. E., Lamppa G. K. Loss of efficient import and thylakoid insertion due to N- and C-terminal deletions in the light-harvesting chlorophyll a/b binding protein. Plant Cell. 1990 Feb;2(2):173–184. doi: 10.1105/tpc.2.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K. Import of proteins into chloroplasts. Membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J Biol Chem. 1986 Nov 5;261(31):14804–14810. [PubMed] [Google Scholar]
- Cline K. Light-Harvesting Chlorophyll a/b Protein : Membrane Insertion, Proteolytic Processing, Assembly into LHC II, and Localization to Appressed Membranes Occurs in Chloroplast Lysates. Plant Physiol. 1988 Apr;86(4):1120–1126. doi: 10.1104/pp.86.4.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller B. L., Wickner W. M13 procoat inserts into liposomes in the absence of other membrane proteins. J Biol Chem. 1985 Oct 25;260(24):13281–13285. [PubMed] [Google Scholar]
- Green B. R., Pichersky E., Kloppstech K. Chlorophyll a/b-binding proteins: an extended family. Trends Biochem Sci. 1991 May;16(5):181–186. doi: 10.1016/0968-0004(91)90072-4. [DOI] [PubMed] [Google Scholar]
- Hand J. M., Szabo L. J., Vasconcelos A. C., Cashmore A. R. The transit peptide of a chloroplast thylakoid membrane protein is functionally equivalent to a stromal-targeting sequence. EMBO J. 1989 Nov;8(11):3195–3206. doi: 10.1002/j.1460-2075.1989.tb08478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin-Neumann G. A., Kohorn B. D., Thornber J. P., Tobin E. M. A chlorophyll a/b-protein encoded by a gene containing an intron with characteristics of a transposable element. J Mol Appl Genet. 1985;3(1):45–61. [PubMed] [Google Scholar]
- Kohorn B. D., Harel E., Chitnis P. R., Thornber J. P., Tobin E. M. Functional and mutational analysis of the light-harvesting chlorophyll a/b protein of thylakoid membranes. J Cell Biol. 1986 Mar;102(3):972–981. doi: 10.1083/jcb.102.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn B. D., Tobin E. M. A hydrophobic, carboxy-proximal region of a light-harvesting chlorophyll a/b protein is necessary for stable integration into thylakoid membranes. Plant Cell. 1989 Jan;1(1):159–166. doi: 10.1105/tpc.1.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühlbrandt W., Wang D. N. Three-dimensional structure of plant light-harvesting complex determined by electron crystallography. Nature. 1991 Mar 14;350(6314):130–134. doi: 10.1038/350130a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamppa G. K. The chlorophyll a/b-binding protein inserts into the thylakoids independent of its cognate transit peptide. J Biol Chem. 1988 Oct 15;263(29):14996–14999. [PubMed] [Google Scholar]
- Payan L. A., Cline K. A stromal protein factor maintains the solubility and insertion competence of an imported thylakoid membrane protein. J Cell Biol. 1991 Feb;112(4):603–613. doi: 10.1083/jcb.112.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. E., Cline K., Stephens L. C., Bacot K. O., Viitanen P. V. Early events in the import/assembly pathway of an integral thylakoid protein. Eur J Biochem. 1990 Nov 26;194(1):33–42. doi: 10.1111/j.1432-1033.1990.tb19423.x. [DOI] [PubMed] [Google Scholar]
- Rohrer J., Kuhn A. The function of a leader peptide in translocating charged amino acyl residues across a membrane. Science. 1990 Dec 7;250(4986):1418–1421. doi: 10.1126/science.2124001. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Smeekens S., Weisbeek P., Robinson C. Protein transport into and within chloroplasts. Trends Biochem Sci. 1990 Feb;15(2):73–76. doi: 10.1016/0968-0004(90)90180-j. [DOI] [PubMed] [Google Scholar]
- Viitanen P. V., Doran E. R., Dunsmuir P. What is the role of the transit peptide in thylakoid integration of the light-harvesting chlorophyll a/b protein? J Biol Chem. 1988 Oct 15;263(29):15000–15007. [PubMed] [Google Scholar]
- Wickner W., Driessen A. J., Hartl F. U. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu Rev Biochem. 1991;60:101–124. doi: 10.1146/annurev.bi.60.070191.000533. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature. 1989 Oct 5;341(6241):456–458. doi: 10.1038/341456a0. [DOI] [PubMed] [Google Scholar]