Table 1. Direct Amidation of Diamine with (R)-Pantalactonea.
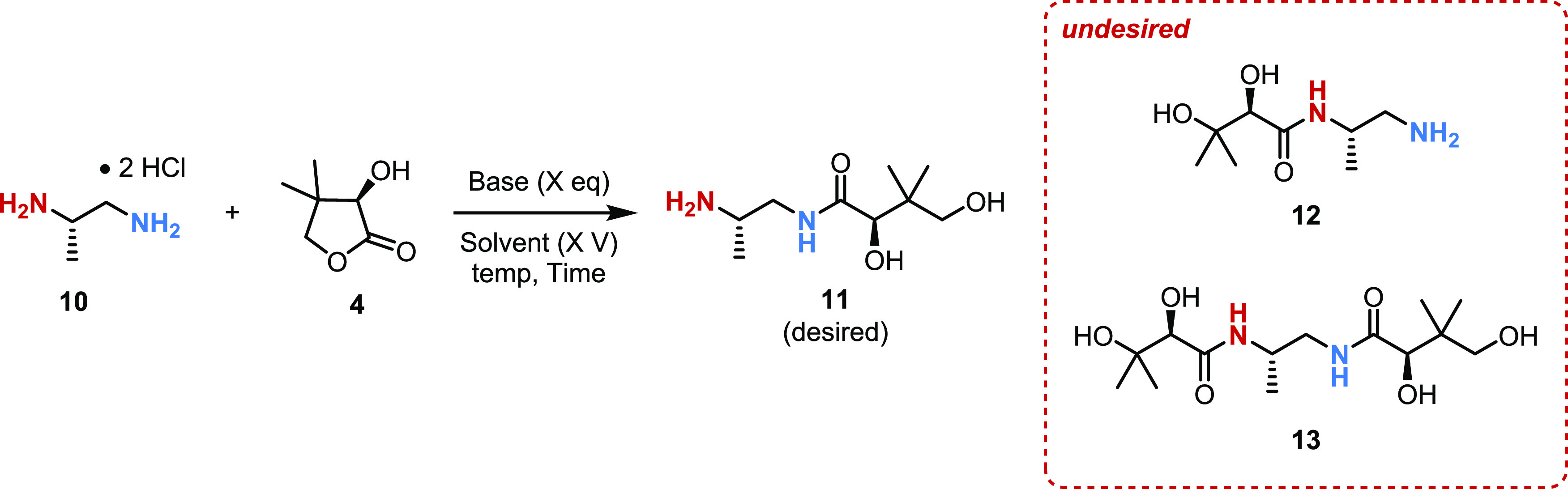
| HPLC
(area %)b |
HILIC (area % ratio)c | ||||||
|---|---|---|---|---|---|---|---|
| entry | solvent | volume (V) | base | T (h) | 13 | 11 + 12 | 11:12 |
| 1 | EtOH | 10 | Na2CO3 | 3 | 8 | 52 | 89:11 |
| 2 | MeOH | 10 | Na2CO3 | 3 | 4 | 82 | 90:10 |
| 3 | IPA | 10 | Na2CO3 | 72 | 8 | 41 | 90:10 |
| 4 | THF | 10 | Na2CO3 | 3 | --e | -- | -- |
| 5 | CH3CN | 10 | Na2CO3 | 3 | --e | -- | -- |
| 6 | DMF | 10 | Na2CO3 | 3 | --e | -- | -- |
| 7 | DMSO | 10 | Na2CO3 | 3 | --e | -- | -- |
| 8 | IPA/H2O (7:3)d | 10 | Na2CO3 | 3 | 5 | 86 | 91:09 |
| 9 | IPA/H2O (7:3) | 5 | Na2CO3 | 3 | 7 | 84 | 90:10 |
| 10 | IPA/H2O (7:3) | 20 | Na2CO3 | 3 | 6 | 84 | 92:08 |
| 11 | IPA/H2O (7:3) | 10 | Et3N | 3 | 6 | 88 | 91:09 |
| 12 | IPA/H2O (7:3) | 10 | NaHCO3 | 3 | --e | -- | -- |
| 13 | IPA/H2O (7:3) | 10 | NaOCH3 | 6 | 45 | 55 | 90:10 |
| 14 | IPA/H2O (9:1) | 10 | Na2CO3 | 3 | 4 | 86 | 90:10 |
| 15f | IPA/H2O (9:1) | 10 | Na2CO3 | 6 | 11 | 86 | 90:10 |
| 16g | IPA/H2O (9:1) | 10 | Na2CO3 | 6 | 5 | 81 | 90:10 |
| 17h | IPA/H2O (9:1) | 10 | Na2CO3 | 6 | 6 | 83 | 90:10 |
All reactions were carried out with (S)-propane-1,2-diamine dihydrochloride 10 (1.0 g 1.0 equiv), (R)-3-hydroxy-4,4-dimethyldihydrofuran-2(3H)-one 4 (1.0 equiv), base (3,0 equiv), 25 °C, 10 V of solvent, unless otherwise stated.
LCAP at 210 nm.
HILIC ratio at 210 nm.
IPA: i-PrOH.
No reaction.
Reaction was carried out at 0 °C.
(2.0 equiv) of Na2CO3 was used.
(2.5 equiv) of Na2CO3 was used.
