Table 2. Purification of 11:12 Monoamide Mixturea.
| HILIC (A % ratio)b | ||||
|---|---|---|---|---|
| entry | acid | solvent | result | 11′:12′ |
| 1 | 4.0 M HCl | 1,4-dioxane | amide cleaved | ND |
| 2 | 1.2 M HCl | IPA | amide cleaved | ND |
| 3 | H2SO4 | EtOH | amide cleaved | ND |
| 4 | H3PO4 | EtOH | no precipitate | NA |
| 5 | formic acid | EtOH | no precipitate | NA |
| 6 | benzoic acid | EtOH | no precipitate | NA |
| 7 | citric acid | EtOH | no precipitate | NA |
| 8 | d-(−)-tartaric acid | EtOH | no precipitate | NA |
| 9c | l-(+)-tartaric acid | EtOH | stable white salt | 25:1 |
| 10 | l-(+)-tartaric acid | Methanol | no precipitate | NA |
| 11 | l-(+)-tartaric acid | Acetone | no precipitate | NA |
| 12 | l-(+)-tartaric acid | EtOAc | no precipitate | NA |
| 13d | l-(+)-tartaric acid | i-PrOH | hygroscopic white salt | 98:2 |
| 14d | l-(+)-tartaric acid | i-PrOH/MeOH (9:1) | stable white salt | 100:0 |
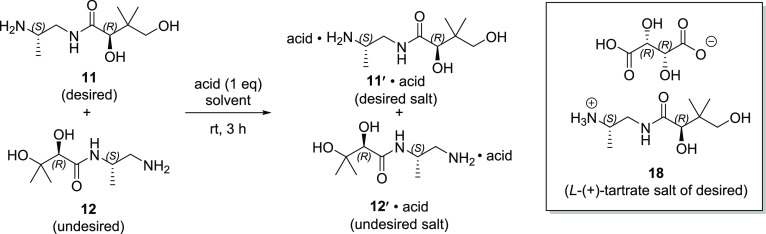
All of the reactions were carried out with ∼9:1 regioisomeric mixture of 11:12 (1.0 g, 1.0 equiv), acid (1.0 equiv), solvent (10 V), rt, 3h.
HILIC ratio at 210 nm. ND = Not determined. NA = Not applicable.
80% of mass recovery.
78% of isolated yield.