Abstract
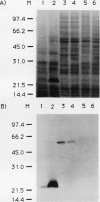
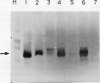
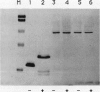
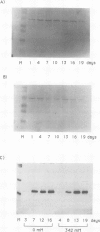
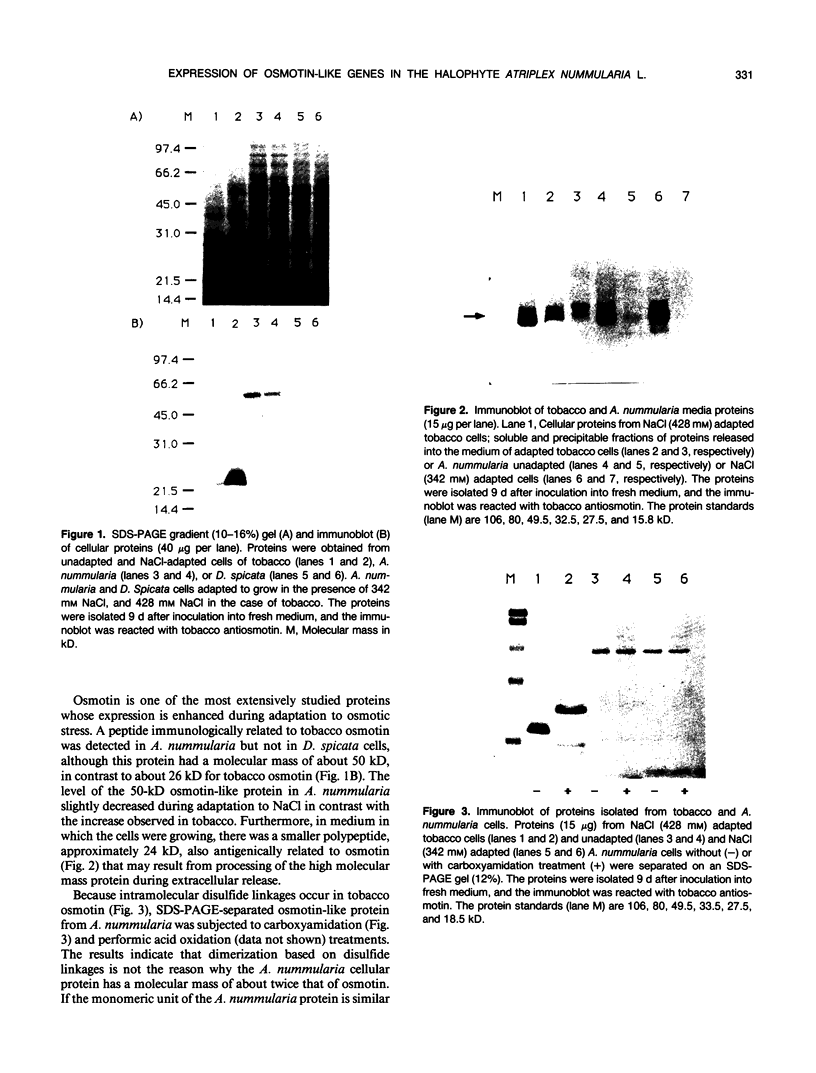
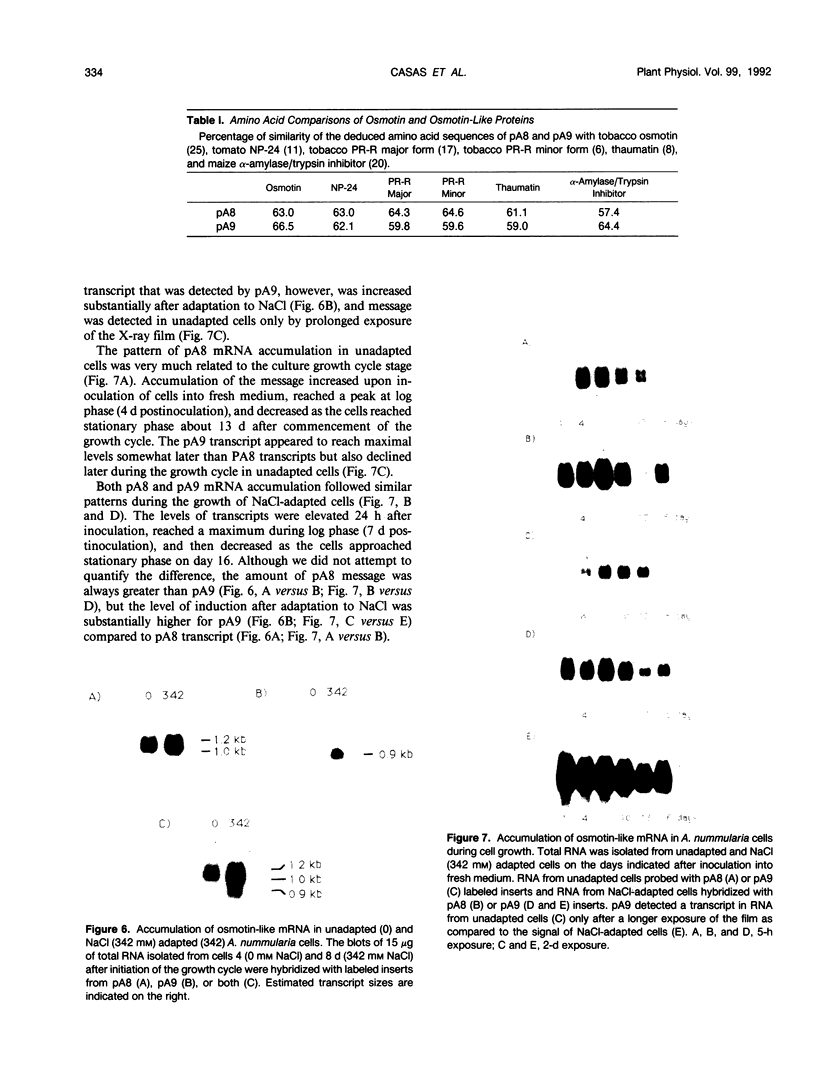
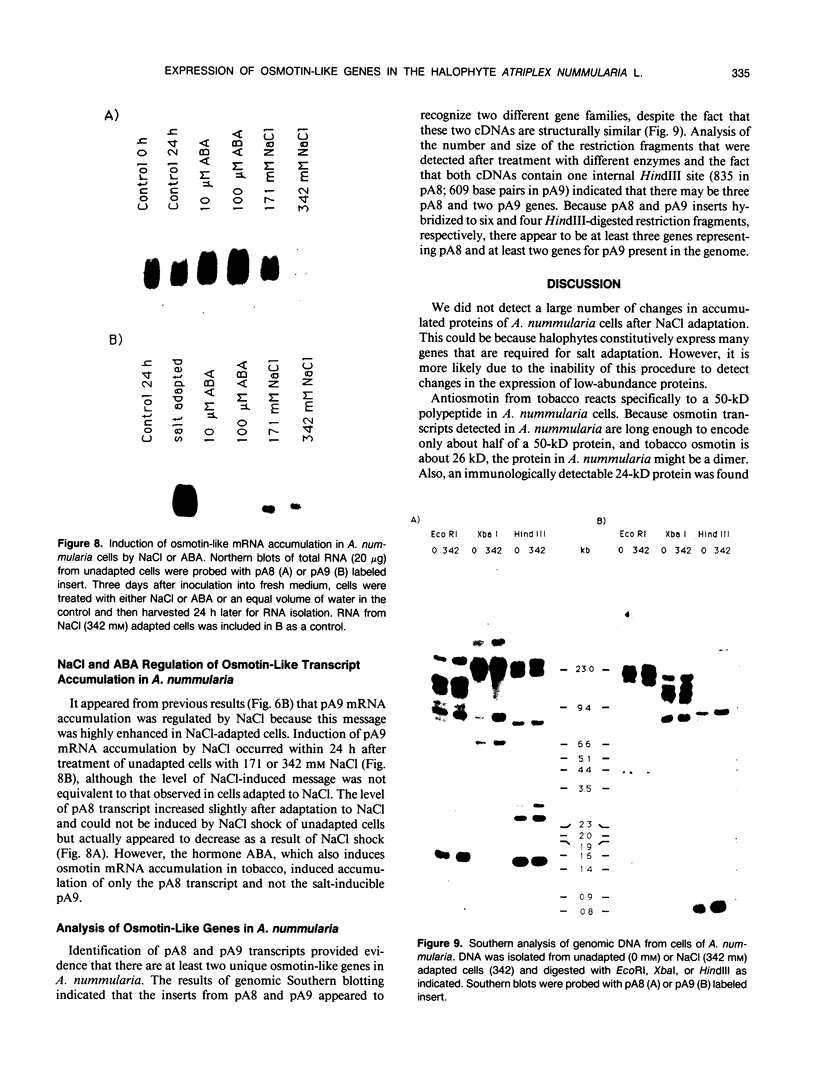
A peptide (molecular mass 50 kilodaltons) that is immunologically related to tobacco osmotin was detected in cells of the halophyte Atriplex nummularia. This protein was constitutively expressed in both unadapted and NaCl-adapted cells. A predominant osmotin-like peptide (molecular mass 24 kilodaltons) was also found in culture media after cell growth. Two unique A. nummularia cDNA clones, pA8 and pA9, encoding osmotin-like proteins have been isolated. The pA8 and pA9 inserts are 952 and 792 base pairs and encode peptides of 222 and 224 amino acids, respectively. The peptide deduced from pA8 has a molecular mass of 23,808 daltons and theoretical isoelectric point of 8.31, whereas the peptide derived from pA9 has a molecular mass of 23,827 daltons and an isoelectric point of 6.88. Unique transcripts were detected by the inserts of the cDNA clones, two (1.2 and 1.0 kilobases) by pA8 and one (0.9 kilobase) by pA9. The pA8 transcripts were constitutively accumulated in unadapted and NaCl-adapted cells, whereas the mRNA levels were up-regulated by abscisic acid treatment. The level of pA9 mRNA was induced by NaCl treatment and increased in cells as a function of NaCl adaptation. Southern analysis of the genomic DNA indicated the presence of osmotin-like multigene families in A. nummularia.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binzel M. L., Hasegawa P. M., Handa A. K., Bressan R. A. Adaptation of Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):118–125. doi: 10.1104/pp.79.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Claes B., Dekeyser R., Villarroel R., Van den Bulcke M., Bauw G., Van Montagu M., Caplan A. Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell. 1990 Jan;2(1):19–27. doi: 10.1105/tpc.2.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen B. J., Hooft van Huijsduijnen R. A., Bol J. F. A tobacco mosaic virus-induced tobacco protein is homologous to the sweet-tasting protein thaumatin. 1986 May 29-Jun 4Nature. 321(6069):531–532. doi: 10.1038/321531a0. [DOI] [PubMed] [Google Scholar]
- Edens L., Heslinga L., Klok R., Ledeboer A. M., Maat J., Toonen M. Y., Visser C., Verrips C. T. Cloning of cDNA encoding the sweet-tasting plant protein thaumatin and its expression in Escherichia coli. Gene. 1982 Apr;18(1):1–12. doi: 10.1016/0378-1119(82)90050-6. [DOI] [PubMed] [Google Scholar]
- Larosa P. C., Singh N. K., Hasegawa P. M., Bressan R. A. Stable NaCl Tolerance of Tobacco Cells Is Associated with Enhanced Accumulation of Osmotin. Plant Physiol. 1989 Nov;91(3):855–861. doi: 10.1104/pp.91.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy J., Chua N. H. Abscisic acid and water-stress induce the expression of a novel rice gene. EMBO J. 1988 Aug;7(8):2279–2286. doi: 10.1002/j.1460-2075.1988.tb03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale A. D., Wahleithner J. A., Lund M., Bonnett H. T., Kelly A., Meeks-Wagner D. R., Peacock W. J., Dennis E. S. Chitinase, beta-1,3-glucanase, osmotin, and extensin are expressed in tobacco explants during flower formation. Plant Cell. 1990 Jul;2(7):673–684. doi: 10.1105/tpc.2.7.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatkowski D., Schneider K., Salamini F., Bartels D. Characterization of Five Abscisic Acid-Responsive cDNA Clones Isolated from the Desiccation-Tolerant Plant Craterostigma plantagineum and Their Relationship to Other Water-Stress Genes. Plant Physiol. 1990 Dec;94(4):1682–1688. doi: 10.1104/pp.94.4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Bracker C. A., Hasegawa P. M., Handa A. K., Buckel S., Hermodson M. A., Pfankoch E., Regnier F. E., Bressan R. A. Characterization of osmotin : a thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987 Oct;85(2):529–536. doi: 10.1104/pp.85.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Handa A. K., Hasegawa P. M., Bressan R. A. Proteins Associated with Adaptation of Cultured Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):126–137. doi: 10.1104/pp.79.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Larosa P. C., Handa A. K., Hasegawa P. M., Bressan R. A. Hormonal regulation of protein synthesis associated with salt tolerance in plant cells. Proc Natl Acad Sci U S A. 1987 Feb;84(3):739–743. doi: 10.1073/pnas.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Nelson D. E., Kuhn D., Hasegawa P. M., Bressan R. A. Molecular Cloning of Osmotin and Regulation of Its Expression by ABA and Adaptation to Low Water Potential. Plant Physiol. 1989 Jul;90(3):1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigers A. J., Roberts W. K., Selitrennikoff C. P. A new family of plant antifungal proteins. Mol Plant Microbe Interact. 1991 Jul-Aug;4(4):315–323. doi: 10.1094/mpmi-4-315. [DOI] [PubMed] [Google Scholar]